People often hear that generic drugs are just copies - cheap knockoffs with less punch. But that’s not true. If you’ve ever been handed a generic pill and wondered if it’s really the same as the brand-name version you’ve been taking, you’re not alone. The truth? Generic drugs aren’t copies in the way a fake handbag is a copy. They’re scientifically proven to work the same way, at the same strength, and with the same results - and they’ve been rigorously tested to prove it.
What Exactly Makes a Generic Drug a Generic Drug?
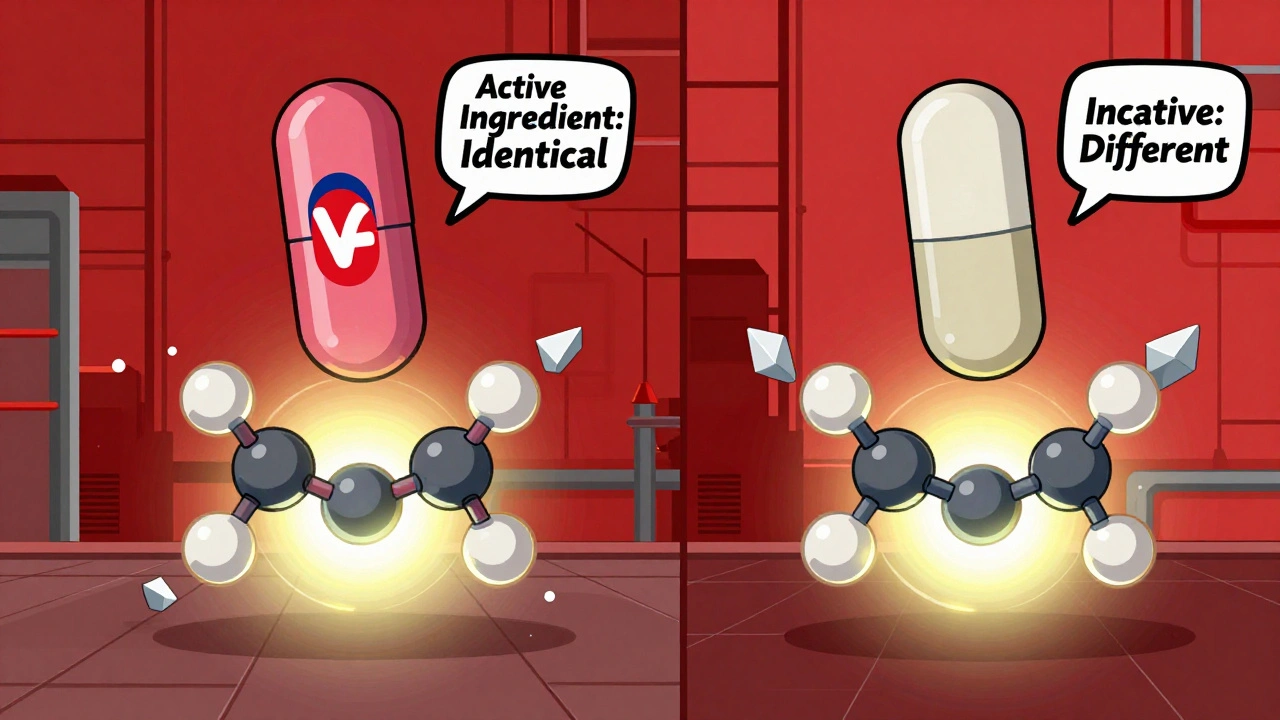
A generic drug must contain the exact same active ingredient as the brand-name version. That means if your brand-name pill has 10 milligrams of atorvastatin, the generic has 10 milligrams of atorvastatin - no more, no less. The FDA requires this. Not just a close guess. Not ‘about the same.’ Exactly the same.
It also has to be the same strength, the same form (tablet, capsule, liquid), and the same way it’s taken (by mouth, injected, applied to skin). The label on the bottle? Identical in terms of dosage instructions, warnings, and how it works in your body.
The only things that can be different are the inactive ingredients - the fillers, dyes, flavors, and preservatives. That’s why a generic version of a blue pill might be white and round instead of blue and oval. It’s not a different drug. It’s just dressed differently. Think of it like two different brands of aspirin: one has a coating to make it easier to swallow, the other doesn’t. Both still relieve headaches the same way.
How Do We Know Generics Work the Same?
The FDA doesn’t just take a manufacturer’s word for it. Every generic drug has to pass a test called bioequivalence. That means scientists measure how much of the active ingredient enters your bloodstream and how fast it gets there. The generic must deliver the same amount of medicine, at the same speed, as the brand-name drug.
The standard? The amount of drug in your blood must fall within 80% to 125% of the brand-name version. That’s not a wide gap - it’s tighter than the tolerance for many everyday products. For example, if your brand-name drug delivers 100 units of medicine into your blood, the generic can’t deliver less than 80 or more than 125. In real-world testing, most generics land within 95% to 105% - nearly identical.
This isn’t a one-time check. The FDA inspects manufacturing sites, reviews batch-to-batch consistency, and monitors the drug after it hits the market. Between 2018 and 2022, the FDA reviewed over 1,800 reports of possible issues with generic drugs. Only 5.5 cases per year were confirmed as actual bioequivalence failures - less than 0.3% of all reports.
Why Are Generics So Much Cheaper?
Generic drugs cost, on average, 85% less than their brand-name counterparts. A prescription that costs $62 for the brand name might be $4.27 as a generic. That’s not because the generic is weaker. It’s because the company making it didn’t have to spend hundreds of millions on research, clinical trials, and marketing.
When a brand-name drug’s patent expires - usually 20 years after it’s filed - other companies can step in and make the same medicine. They don’t need to repeat expensive human trials because they’re proving equivalence, not starting from scratch. The system was designed this way by the Hatch-Waxman Act of 1984 to open up competition and bring down prices.
Today, 90% of all prescriptions filled in the U.S. are for generic drugs. Yet they make up only about 23% of total drug spending. That’s billions saved every year - for patients, insurers, and taxpayers.
Are There Times When Generics Aren’t the Best Choice?
Yes - but they’re rare. For most medications, switching from brand to generic is safe and effective. But there’s a small group of drugs called narrow therapeutic index (NTI) drugs. These are medicines where even tiny changes in blood levels can cause problems - either the drug stops working, or it becomes dangerous.
Examples include warfarin (a blood thinner), levothyroxine (for thyroid conditions), and certain seizure medications like phenytoin. For these, doctors and pharmacists may recommend sticking with the same brand - not because generics are inferior, but because consistency matters more than cost. If you’ve been stable on a brand-name version for years, switching might require extra monitoring.
Even then, studies show that 92% of patients on NTI drugs can safely switch to generics with proper oversight. The Epilepsy Foundation found that 17% of patients reported breakthrough seizures after switching - but follow-up analysis by the FDA found most cases were linked to other factors like missed doses, illness, or stress, not the drug itself.
What Do Real Patients Say?
On Drugs.com, over 1.2 million reviews of generic medications show an average rating of 7.2 out of 10. Brand-name drugs? 7.5. That’s a tiny difference - and most of it comes from people who just prefer the look or feel of the original pill.
Reddit’s r/pharmacy community tracked over 4,000 discussions about generic switches. Two-thirds of users reported no difference at all. A quarter said they noticed minor side effects - like a stomachache or dizziness - but those often went away after a few days. In most cases, it wasn’t the active ingredient causing the issue. It was a new filler or coating they weren’t used to.
A Kaiser Family Foundation survey found that 78% of insured adults get generics as their first prescription. Of those, 89% were satisfied. Medicare Part D users saved over $500 a year on average just by using generics.
Can a Pharmacist Switch My Prescription Without Asking?
In 49 states, yes. Pharmacists are allowed to substitute a generic for a brand-name drug unless the doctor writes ‘dispense as written’ or ‘no substitution.’ In Mississippi, they need the doctor to sign off on the substitution.
But here’s the thing: you can always ask for the brand name. If your insurance covers the generic, they’ll usually charge you the generic price even if you get the brand. Some states even require pharmacies to tell you this option exists.
Still, 65% of patients ask for brand-name drugs because they think generics are weaker. That’s a myth. FDA testing shows generics contain, on average, 99.2% of the active ingredient listed on the label - almost identical to brand-name drugs.
What’s Changing in the Generic Drug World?
The FDA is pushing to cut approval times for generics from over three years down to 10 months by 2027. Right now, there are over 1,200 complex generics waiting for approval - things like inhalers, eye drops, and topical creams that are harder to copy because they don’t dissolve the same way in the body.
At the same time, biosimilars - the next generation of generics for biologic drugs like Humira or Enbrel - are starting to hit the market. These aren’t exact copies (because biologics are made from living cells), but they’re proven to work just as well. By 2027, they could make up 15% of the market.
And thanks to new Medicare rules, generics will now be automatically substituted unless a doctor says otherwise - saving an estimated $156 billion over the next decade.
Bottom Line: Generics Are Not Copies. They’re Equivalents.
Generic drugs are not cheap imitations. They’re scientifically validated, FDA-approved, and just as effective as their brand-name counterparts for nearly every condition. The only real difference? Price.
If you’re worried about switching, talk to your pharmacist. They can explain why your generic looks different, what’s in it, and whether it’s right for you. For most people, the switch is seamless. For the few who need extra care - like those on blood thinners or seizure meds - your doctor will know.
Don’t let the color or shape fool you. The medicine inside? It’s the same.
Are generic drugs just as effective as brand-name drugs?
Yes. The FDA requires generic drugs to have the same active ingredient, strength, dosage form, and route of administration as the brand-name version. They must also prove bioequivalence - meaning they deliver the same amount of medicine into your bloodstream at the same rate. For over 90% of medications, generics work just as well.
Why do generic drugs look different from brand-name drugs?
U.S. trademark laws require generic drugs to look different from brand-name versions. That means the color, shape, size, or flavor can change. But these are all inactive ingredients - they don’t affect how the drug works. Only the active ingredient matters for effectiveness.
Can I trust generics if they’re so much cheaper?
Absolutely. Lower cost doesn’t mean lower quality. Generic manufacturers don’t have to repeat expensive clinical trials because they’re proving equivalence, not inventing something new. The FDA inspects their factories just like brand-name ones. In fact, many brand-name companies make their own generics under different labels.
Are there any drugs where I should avoid generics?
For most drugs, no. But for narrow therapeutic index (NTI) drugs - like warfarin, levothyroxine, and some seizure medications - small changes in blood levels can matter. In these cases, your doctor might recommend sticking with one brand for consistency. That doesn’t mean generics are unsafe - just that monitoring is important.
Can my pharmacist switch my prescription to a generic without telling me?
In most states, yes - but only if your doctor didn’t write ‘dispense as written.’ Pharmacists are required to offer a generic if one is available and approved. You can always ask for the brand name, and in many cases, you’ll still pay the generic price. If you’re unsure, just ask your pharmacist to explain what you’re getting.
Do generics have more side effects?
Not because of the active ingredient. Any side effects you notice after switching are usually due to changes in inactive ingredients - like a new filler or coating. These are rare and often temporary. If you feel different after switching, talk to your pharmacist or doctor. It’s not the medicine failing - it’s your body adjusting.
How do I know if my generic drug is FDA-approved?
All approved generic drugs are listed in the FDA’s Orange Book. You can ask your pharmacist for the name of the manufacturer and look it up. If it’s on the market and sold in the U.S., it’s been reviewed and approved. There’s no such thing as an unapproved generic sold legally in pharmacies.
Why do some people say generics don’t work for them?
Sometimes, it’s psychological - they expect a difference because it looks different. Other times, it’s a change in inactive ingredients causing temporary discomfort. Rarely, it’s a true issue with an NTI drug that needs closer monitoring. But for the vast majority, the problem isn’t the drug - it’s the misunderstanding. Studies show 82% of users report identical effectiveness.
sean whitfield
December 6, 2025 AT 03:44Katie Allan
December 7, 2025 AT 20:19Kylee Gregory
December 8, 2025 AT 15:01Lucy Kavanagh
December 9, 2025 AT 14:53Stephanie Fiero
December 10, 2025 AT 19:40Laura Saye
December 12, 2025 AT 11:53Krishan Patel
December 14, 2025 AT 02:48Carole Nkosi
December 14, 2025 AT 12:09Stephanie Bodde
December 15, 2025 AT 11:23