Imagine you fill a prescription for a brand-name drug, and the next time you pick it up, it looks different - different color, different shape, different label. You might think you got the wrong medicine. But here’s the twist: it’s the exact same pill. Same active ingredient. Same factory. Same quality control. The only thing changed? The label.
This isn’t a mistake. It’s an authorized generic.
Authorized generics are the same drug your doctor prescribed, made by the same company that makes the brand-name version, but sold under a generic label. No extra testing. No different formula. Just a cheaper price and a plain box. And while they’ve been around for years, most patients - and even some doctors - don’t know they exist.
How Authorized Generics Work
When a brand-name drug’s patent expires, other companies can make copies. Those are traditional generics. But here’s what most people don’t realize: the original drug company can make its own copy too. That’s an authorized generic.
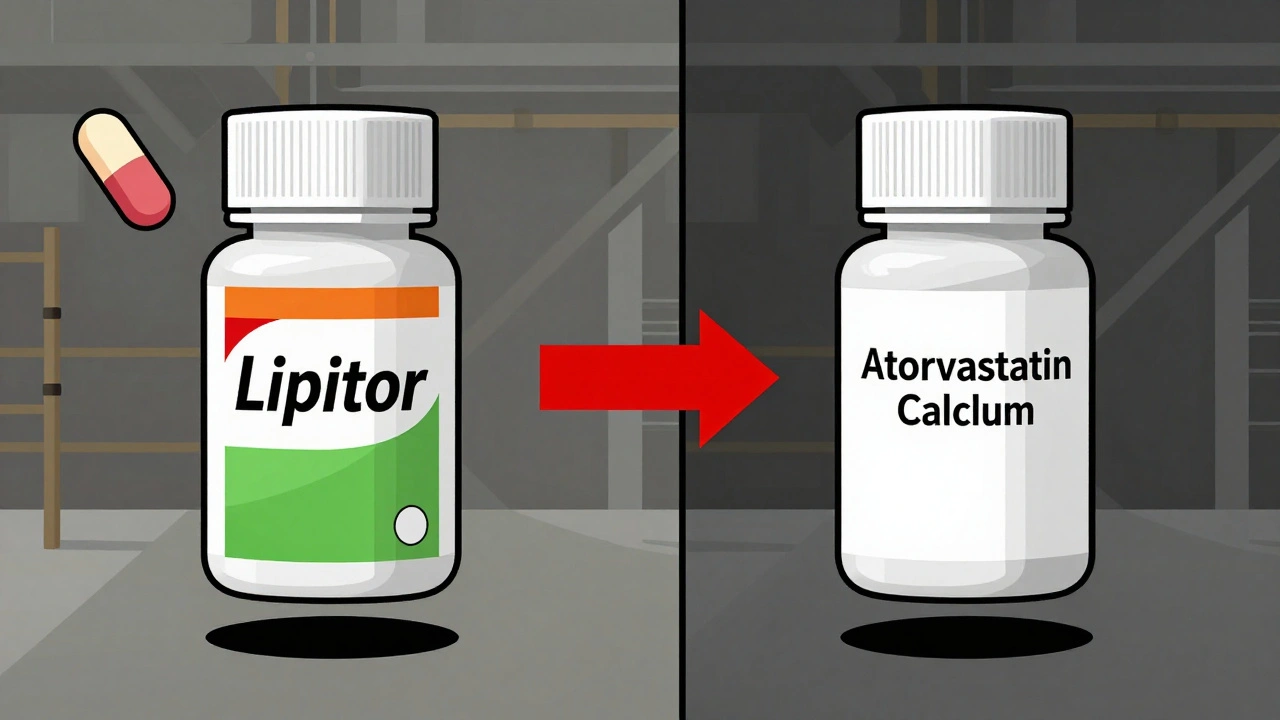
Let’s say you take Lipitor, the brand-name cholesterol drug. Pfizer, the maker, doesn’t just sit back when the patent runs out. Instead, they launch a version of Lipitor under a different name - say, “Atorvastatin Calcium” - with no brand logo, no fancy packaging, and a lower price. But it’s the exact same tablet, made on the same line, with the same inactive ingredients. The FDA calls this an “authorized generic” because it’s produced under the original brand’s approval (NDA), not a new generic application (ANDA).
That’s the key difference. Traditional generics have to prove they work the same way as the brand. Authorized generics don’t. They’re already proven - because they’re the same product.
Why Do Companies Do This?
It’s not charity. It’s strategy.
When the Hatch-Waxman Act passed in 1984, it gave the first company to file a generic version 180 days of exclusive rights. That was meant to encourage competition and lower prices. But big pharma found a loophole. If they launch their own generic version right when the exclusivity period starts, they can undercut the first generic company - often at the same price or even lower.
Companies like Pfizer (through Greenstone), Procter & Gamble (Prasco), and others now have dedicated teams to roll out authorized generics. They’re not trying to help you save money - they’re trying to keep you from switching to someone else’s generic. And it works. In many cases, the authorized generic captures the majority of the market, leaving the first generic company with little to no sales.
For patients, this can mean faster access to lower prices. For the system, it’s messy. Critics say it undermines the whole point of the generic approval system. Supporters say it increases competition and keeps prices down.
Authorized Generic vs. Traditional Generic vs. Brand Name
Here’s how they stack up:
| Feature | Brand Name | Authorized Generic | Traditional Generic |
|---|---|---|---|
| Manufacturer | Original drug company | Original drug company | Separate generic company |
| Active Ingredient | Identical | Identical | Identical |
| Inactive Ingredients | Brand-specific | Identical to brand | May differ |
| Regulatory Path | New Drug Application (NDA) | Uses brand’s NDA | Abbreviated NDA (ANDA) |
| Bioequivalence Required? | No - it’s the original | No - same as brand | Yes - must prove equivalence |
| Appears in FDA Orange Book? | Yes | No | Yes |
| Typical Price | Highest | Low - often same as traditional generic | Low |
Notice something? Authorized generics are the only type that’s 100% identical to the brand - down to the inactive ingredients. Traditional generics only need to match the active ingredient. That means sometimes, a traditional generic might cause a slightly different reaction in people who are sensitive to dyes, fillers, or coatings. Authorized generics? No risk there.
What You’ll See When You Get One
When you pick up your prescription and the pill looks different, don’t panic. That’s normal. Authorized generics often have a different color, imprint, or shape - just enough to tell them apart from the brand. But they’re not a different drug.
Some patients report confusion. One woman in Toronto told her pharmacist she refused to take her new pills because they were “clear” instead of “blue.” She was on the same dose of metformin, just an authorized generic. Her doctor had no idea it had switched. Her pharmacist had to call the manufacturer to confirm it was safe.
Pharmacists face challenges too. Authorized generics don’t show up in the FDA’s Orange Book, so they can’t be automatically substituted like traditional generics. That means the pharmacist has to manually verify it’s the right drug - often by checking the FDA’s separate list of authorized generics or calling the manufacturer.
Are Authorized Generics Safe?
Yes. Absolutely.
The FDA considers them therapeutically equivalent to the brand. In fact, they’re more equivalent than traditional generics - because they’re literally the same product. There’s no extra risk. No hidden ingredients. No compromise in quality.
And here’s the kicker: many authorized generics are made in the same facility as the brand-name version. Same machines. Same inspectors. Same batch records. The only difference? The label says “Atorvastatin” instead of “Lipitor.”
If you’ve ever worried that generics are “weaker” or “less effective,” authorized generics prove that’s not true. They’re the original - just cheaper.
How to Know If You’re Getting One
You won’t always know. But here’s how to find out:
- Check the label. If it has the drug name (like “Metformin”) but no brand name (like “Glucophage”), it might be generic - but not necessarily authorized.
- Ask your pharmacist: “Is this an authorized generic?” They can check the FDA’s list or the manufacturer’s info.
- Look up the pill imprint on a site like Drugs.com or WebMD. If the description says “manufactured by [Brand Name]” - that’s an authorized generic.
- Compare the price. If it’s much cheaper than the brand but the same as other generics, it’s likely authorized.
Some pharmacies automatically switch you to the lowest-cost option - which could be a traditional generic or an authorized one. You have the right to ask for the brand if you prefer. But if you’re okay with the generic, and the price is low, go for it.
Why This Matters for Your Wallet
Brand-name drugs can cost hundreds of dollars a month. Traditional generics? Often $10-$20. Authorized generics? Sometimes even less - $5, $3, or free with coupons.
One study found that when an authorized generic entered the market, the price of the brand dropped by 40% within months. The traditional generic price dropped too. Everyone got cheaper.
That’s not luck. It’s competition. And authorized generics are a big part of that.
If you’re paying full price for a brand-name drug, ask your doctor or pharmacist: “Is there an authorized generic for this?” You might be leaving money on the table.
What’s Next for Authorized Generics?
More drugs are losing patents every year. In 2025 alone, over 30 major brand-name drugs will go generic in the U.S. Many of those will have authorized versions released by the original manufacturers.
There’s debate in Congress about whether this practice should be limited. Some lawmakers argue it stifles true generic competition. Others say it’s a win for patients - more choices, lower prices, same quality.
For now, authorized generics are here to stay. And if you’re on a chronic medication, you’re likely to encounter one soon.
Don’t fear the different-looking pill. It’s not a downgrade. It’s a smarter way to get the same medicine - for less.
Are authorized generics the same as brand-name drugs?
Yes. Authorized generics are identical to their brand-name counterparts in active ingredients, dosage, strength, safety, and effectiveness. They’re made in the same facility, using the same formula and manufacturing process. The only differences are the label, packaging, and price.
Why do authorized generics cost less than brand-name drugs?
They cost less because they don’t need to cover the high costs of research, marketing, and advertising that brand-name drugs do. The manufacturer already recovered those costs during the patent period. With an authorized generic, they’re simply selling the same product under a cheaper label - no extra expenses.
Can I trust an authorized generic as much as the brand?
Absolutely. The FDA requires that authorized generics meet the same quality standards as the brand. Since they’re made by the same company using the same process, there’s no reason to expect any difference in safety or effectiveness. Many patients report no change in how they feel when switching.
Why don’t pharmacists always tell me I’m getting an authorized generic?
Many pharmacists aren’t trained to distinguish between traditional generics and authorized generics. Authorized generics don’t appear in the FDA’s Orange Book, so they’re not flagged in pharmacy systems. If you want to know, ask directly: “Is this an authorized generic?”
Do authorized generics affect insurance coverage?
Usually not. Most insurance plans treat authorized generics the same as traditional generics - meaning you pay your usual generic copay. Some plans even prefer them because they’re cheaper than the brand. Always check your plan’s formulary, but in most cases, switching won’t change your out-of-pocket cost.
If you’re on a long-term medication, ask your pharmacist to check if an authorized generic is available. You might be paying more than you need to - for the exact same pill.
Kristi Pope
December 11, 2025 AT 16:02So basically if you’re on a drug that’s been around for a while and suddenly your pill looks like it came from a discount store, don’t freak out-it’s the same thing, just cheaper. I switched my metformin to an authorized generic last year and didn’t even notice until I checked the label. Saved me $40 a month. Why isn’t this common knowledge?
Neelam Kumari
December 12, 2025 AT 03:46Wow. So the same company that gouged us for 15 years now wants us to cheer because they’re selling the same pill for $3? Genius. Next they’ll patent the bottle and charge us extra for the cap.
Lisa Stringfellow
December 12, 2025 AT 20:28Let me guess-the FDA’s ‘authorized’ stamp means nothing when the company that made the brand also makes the ‘generic’ and still controls the entire supply chain. This isn’t competition. It’s a shell game. I’ve seen people have reactions to ‘identical’ generics because the fillers changed. They just don’t test for that. They test for ‘bioequivalence’-which is a fancy word for ‘close enough.’
And don’t get me started on how pharmacists are left in the dark. My mom got a different pill, panicked, called her doctor, and he said ‘oh yeah, that’s fine’ without checking. That’s not healthcare. That’s negligence wrapped in a corporate strategy.
I’m not anti-generic. I’m pro-transparency. If it’s the same pill, why hide it? Why not label it clearly? Why make people Google their pill imprint like it’s a mystery box?
And yes, I know it’s cheaper. But I’d rather pay more for a system that doesn’t treat me like a number in a profit algorithm.
They’re not lowering prices to help us. They’re lowering them to kill the competition. And then they’ll raise them again when there’s no one left to challenge them.
I’ve seen this movie before. Remember the EpiPen? Same playbook. Same actors. Different drug.
They’re not heroes. They’re strategists. And we’re the pawns.
Next time your prescription changes, ask: ‘Who really benefits here?’ Not me. Not my health. Just their quarterly earnings.
Queenie Chan
December 12, 2025 AT 23:51Wait, so if the authorized generic is literally the same pill, why doesn’t the pharmacy just say ‘this is the brand, but cheaper’? Why the secrecy? It feels like they’re hiding something, even if it’s not dangerous. Also, why do some people swear their ‘generic’ makes them feel weird but the brand doesn’t? If the active ingredient is the same, why do placebo effects get so real? I’ve had friends swear their blood pressure went haywire on a generic-until they switched back to the brand. Or was it just the label?
Also, does anyone else think the FDA’s ‘authorized’ label is just corporate PR dressed up as regulation? Like, if it’s the same pill, why even have two categories? Why not just say ‘same drug, different price’ and be done with it?
And how do you even know if your pharmacy switched you? I once got a blue pill instead of white, panicked, Googled it, and found out it was the same drug. But I had to do the work. That shouldn’t be on me.
Also, is there a list somewhere that says which drugs have authorized generics? Or do I have to call each manufacturer? This feels like a glitch in the system, not a feature.
Nikki Smellie
December 14, 2025 AT 05:03Are you aware that the FDA’s ‘authorized generic’ list is not publicly searchable? And that the same companies that produce these are also lobbying Congress to block transparency laws? I’ve read the transcripts. They’re terrified of consumers knowing the truth. This isn’t about savings-it’s about control. They want you to think you’re getting a deal… while they quietly eliminate all competition. One day, you’ll wake up and the only option left will be their ‘authorized’ version. And then… they’ll raise the price again. Watch. It’s happening. I’ve been tracking this since 2018. They’re playing a long game. And we’re all just waiting for the next pill to change color.
Monica Evan
December 15, 2025 AT 12:00Just got my authorized generic for lisinopril yesterday-same pill, $3 at CVS. I didn’t even realize it was different until I checked the bottle. My pharmacist didn’t mention it, but I asked because I read about this last year. She said, ‘Oh yeah, that’s the one made by the same company.’ I said ‘cool’ and walked out. No drama. No fear. Just saved money. Why do people make this so complicated? If it’s the same drug, same factory, same quality, why are we treating it like a conspiracy? I’m not saying the system’s perfect, but this? This is actually a win. Stop overthinking it.
Also, I’m Indian-American and my mom’s been on generics for 20 years. She never had an issue. She doesn’t care what the label says as long as it works. Maybe we’re the ones overcomplicating this.
Also, typo: I meant ‘lisinopril’ not ‘lisinopril’-sorry, autocorrect.
Aman deep
December 16, 2025 AT 08:57As someone from India where generics are the norm and brand names are a luxury, this whole thing feels so… American. Here, we don’t care who made it as long as it works. My uncle takes the same blood pressure med for 15 years-never changed brand, never checked label. He just takes it. If it works, it works. If it doesn’t, he goes to the clinic. Simple. No drama. No ‘authorized’ this or ‘traditional’ that. Just medicine. Maybe we’re missing something, but I think the anxiety around this is more about trust in systems than the pills themselves.
Also, I think the real issue isn’t the authorized generic-it’s that we don’t have universal healthcare. If everyone could afford the brand, none of this would matter. But we don’t. So we fight over $3 pills. That’s the tragedy.
And yes, I typoed ‘medicine’ as ‘medicne’-sorry, phone keyboard.
Regan Mears
December 16, 2025 AT 22:12Look-I get why people are suspicious. I was too. But after working in pharmacy for 12 years, I can tell you: authorized generics are the gold standard. Same batch. Same inspector. Same quality control logs. I’ve seen the paperwork. The brand and the authorized generic come off the same line, sometimes within hours of each other. The only difference? The sticker. And yes, pharmacists don’t always know because the Orange Book doesn’t list them-it’s a systemic flaw, not a deception.
But here’s the thing: if you’re worried about inactive ingredients, you should be asking for the authorized generic, not avoiding it. Traditional generics can have different binders, dyes, coatings-those are the ones that cause reactions. Authorized? Zero risk. Same as the brand.
And yes, the pharma companies are playing chess. But guess what? We’re the ones who get to pick up the cheapest, safest option. So why not take it? Don’t fight the system-use it. Ask for it. Demand it. And if your pharmacist doesn’t know? Educate them. I’ve done it. They appreciate it.
This isn’t a loophole. It’s a gift. Use it.
Rebecca Dong
December 18, 2025 AT 02:28So… the FDA lets the same company that made Lipitor sell it as a generic? And no one’s investigating? What if they’re secretly adding something to the brand version to make you dependent? Like, what if the authorized generic is the real drug… and the brand has a hidden additive? Think about it. They control the supply. They control the narrative. They control the labels. They even control the FDA’s list. Who’s auditing them? No one. This is a mind control experiment disguised as healthcare. I’m switching to herbal supplements. At least then I know what’s in it.