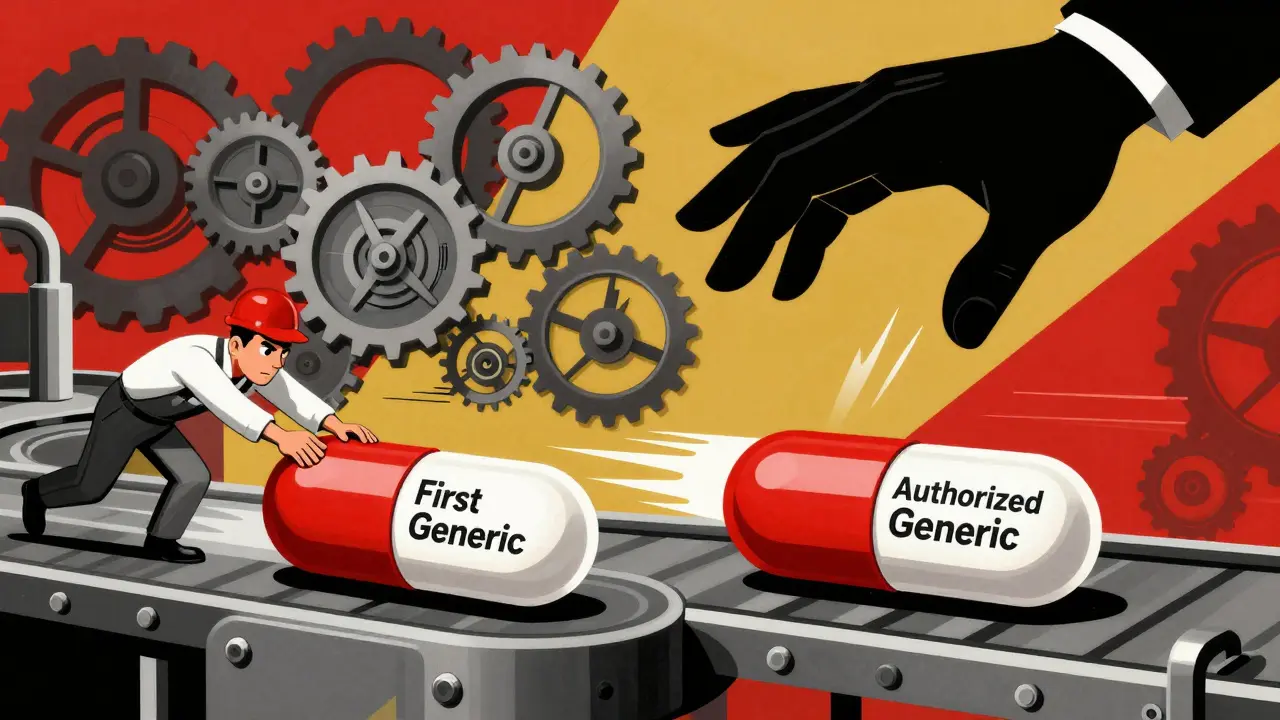
When a brand-name drug loses its patent, the race to sell the first generic version begins. But here’s the twist: the company that made the original drug might launch its own generic version - same pill, same factory, same packaging - just with a cheaper label. This isn’t a mistake. It’s a strategy. And it changes everything about how much you pay for medicine.
What’s the difference between a first generic and an authorized generic?
A first generic is the first company to legally copy a brand-name drug after its patent expires. To do this, they file an Abbreviated New Drug Application (ANDA) with the FDA. They prove their version works the same way as the original. If they’re the first to file and successfully challenge the patent, they get 180 days of exclusive rights to sell the generic. During that time, no other generic can enter the market. That’s a big deal. It means they can capture up to 80% of the market and make millions - sometimes hundreds of millions - before competition kicks in.
An authorized generic is different. It’s made by the brand-name company itself, or by a partner they’ve authorized. It’s chemically identical to the brand drug - same active ingredient, same inactive ingredients, same factory. But it’s sold under a generic name and at a lower price. The key? They don’t need an ANDA. They use the original New Drug Application (NDA) the brand company already has. That means they can launch it in days, not months or years.
Here’s the real kicker: the brand company doesn’t have to wait for the first generic to enter. They can launch their authorized generic the same day - or even before.
Why timing matters more than you think
The 180-day exclusivity period was meant to reward companies that took the risk to challenge expensive patents. But in practice, it’s become a trap.
Take Lyrica (pregabalin). Pfizer’s brand drug was set to lose patent protection in mid-2019. Teva, a major generic maker, spent years preparing its ANDA, investing millions in legal battles and manufacturing setup. When Teva launched its generic in July 2019, they expected to dominate the market. But within hours, Pfizer launched its own authorized generic through Greenstone LLC. Same drug. Same pill. Same manufacturer. Just cheaper.
Result? Teva’s market share dropped from an expected 80% to under 50% in weeks. Pfizer’s authorized generic took about 30% of the market. The rest went to other generics that entered after the exclusivity window. Teva’s profit dropped by nearly half. And patients? They still paid more than they should have.
This isn’t rare. Between 2010 and 2019, 73% of authorized generics launched within 90 days of the first generic. Over 40% launched on the exact same day. That’s not coincidence. That’s a playbook.
How this drives up drug costs
Generic drugs usually cut prices by 80-90%. That’s the whole point. But when an authorized generic enters during the first generic’s exclusivity period, the price drop shrinks to just 65-75%.
Why? Because now there are two sellers - not one. The first generic can’t undercut the authorized generic too much, or they’ll lose money. The authorized generic doesn’t need to compete aggressively - it’s backed by the brand company’s distribution network and brand trust. So prices stay higher than they should.
According to the RAND Corporation, this tactic cost the U.S. healthcare system billions in avoided savings over the past decade. Imagine a $100-a-month drug. Without competition, it stays at $100. With a true first generic, it drops to $20. But with an authorized generic entering right away? It drops to $35. That’s $15 extra per month - $180 a year - for every patient. Multiply that by millions of prescriptions.

Who benefits? Who loses?
Brand-name companies win. They keep control of the market, protect their revenue, and avoid the full sting of generic competition. They get to sell the same product at a lower price - but still make money while keeping rivals at bay.
Authorized generic manufacturers win too. They get access to a proven product without the cost or risk of building their own manufacturing or proving bioequivalence. It’s a low-effort, high-reward play.
First generic companies lose. They invest years and millions into patent challenges, legal fees, and production setup - only to be undercut by the very company they were trying to compete against. Many smaller generic firms have walked away from patent challenges entirely. Why risk $10 million to lose half your profit before you even break even?
Patients and insurers lose. Prices don’t fall as far or as fast. Medicare and Medicaid pay more. Employers pay more for drug coverage. You pay more at the pharmacy counter.
The regulatory blind spot
The FDA approves both types of drugs. But they’re treated differently under the law. The Hatch-Waxman Act of 1984 created the 180-day exclusivity rule to encourage generic competition. But it never accounted for authorized generics.
The FDA doesn’t regulate when a brand company can launch an authorized generic. There’s no rule saying they have to wait. So they don’t. They launch when it hurts the most - right when the first generic is trying to build momentum.
The Inflation Reduction Act of 2022 tried to fix part of this. It said authorized generics don’t count as “generic competitors” when Medicare negotiates drug prices. That means when Medicare tries to lower the cost of a drug like Eliquis or Jardiance, they can’t pretend the authorized generic is helping drive prices down. It’s a small step - but it’s recognition that this isn’t true competition.

What’s changing now?
Generic drug makers are adapting. Some are shifting away from high-risk, high-reward patent challenges. Instead, they’re building portfolios of generic drugs with less competition - or focusing on drugs where the brand company doesn’t have the infrastructure to launch an authorized version.
Others are racing to launch faster. With FDA review times averaging 10 months under GDUFA (and sometimes stretching to three years during backlogs), speed is everything. Companies are investing in pre-filing consultations, parallel manufacturing setups, and legal teams that can move faster.
But here’s the hard truth: if the brand company wants to block you, they can. And they will.
By 2027, experts predict authorized generics will make up 25-30% of all generic prescriptions - up from 18% in 2022. That’s not growth. That’s a transformation of the market.
What you can do
As a patient, you can’t stop a brand company from launching an authorized generic. But you can make smarter choices.
- Ask your pharmacist: “Is this the brand’s authorized generic?” If they say yes, know that the price might not be as low as it could be.
- Compare prices. Sometimes the authorized generic costs more than a non-authorized generic that entered after the exclusivity period.
- Use prescription savings apps. Many offer better deals on non-authorized generics.
- Ask your doctor if a different generic version is available - even if it’s not the first one.
The system is rigged - but not unbeatable. Your awareness is your best tool.
Why this matters beyond the pharmacy
This isn’t just about pills. It’s about how innovation gets rewarded - or punished.
The Hatch-Waxman Act was supposed to balance two things: giving drugmakers time to profit from their inventions, and letting cheaper generics enter quickly to save money. But authorized generics turned that balance into a game of chess - where the brand company always moves first.
If we want real savings, we need rules that treat authorized generics for what they are: a strategic extension of the brand, not true competition. Until then, the savings you expect from generics? They’ll be smaller. Slower. And harder to get.
What’s the difference between a first generic and an authorized generic?
A first generic is the first company to legally copy a brand-name drug after its patent expires. They file an ANDA with the FDA and get 180 days of exclusivity. An authorized generic is made by the brand company or an authorized partner. It’s identical to the brand drug but sold under a generic label. It doesn’t need FDA approval through an ANDA - it uses the brand’s existing NDA, so it can launch instantly.
Why do brand companies launch authorized generics?
They launch them to undercut the first generic’s market share and limit price drops. Instead of letting one generic company capture 80% of the market during its 180-day exclusivity, the brand company splits the market. This keeps prices higher than they’d be with true competition and protects their long-term profits.
Can an authorized generic enter before the first generic?
Yes. Authorized generics can launch anytime after the brand drug’s patent expires - even before the first generic is approved. The brand company doesn’t need to wait for FDA approval of a generic application. They control their own supply chain and can switch labels overnight.
Do authorized generics lower drug prices?
They lower prices a little - but not as much as true generic competition. When an authorized generic enters during the first generic’s exclusivity period, prices typically drop only 65-75%, not the 80-90% seen with unchallenged generic entry. That’s because two sellers are sharing the market, not one.
Are authorized generics the same as the brand drug?
Yes. Authorized generics are chemically identical to the brand drug - same active ingredient, same inactive ingredients, same manufacturing facility. The only difference is the label and the price.
Is it legal for brand companies to launch authorized generics?
Yes. There’s no law preventing it. The FDA allows it, and courts have upheld the practice. But it’s controversial. Critics say it undermines the Hatch-Waxman Act’s goal of encouraging real generic competition. Some lawmakers are pushing for reforms to limit this tactic.
Susan Arlene
January 4, 2026 AT 23:08Leonard Shit
January 6, 2026 AT 19:27Mukesh Pareek
January 8, 2026 AT 10:16Joann Absi
January 8, 2026 AT 20:52Rachel Wermager
January 9, 2026 AT 03:31Indra Triawan
January 9, 2026 AT 09:39Ashley S
January 10, 2026 AT 18:19Vinayak Naik
January 11, 2026 AT 14:28Gabrielle Panchev
January 11, 2026 AT 14:46Harshit Kansal
January 12, 2026 AT 05:37Lily Lilyy
January 13, 2026 AT 16:31Melanie Clark
January 15, 2026 AT 14:45Katelyn Slack
January 15, 2026 AT 22:18