Have you ever noticed that the same generic pill you take in the U.S. costs a fraction of what it does in Switzerland-or even in Canada? It’s not a glitch. It’s the reality of how generic drugs are made, sold, and regulated across the world. The same active ingredient, the same dosage, the same manufacturer-but wildly different prices, availability, and even patient experiences. This isn’t about quality control alone. It’s about laws, markets, politics, and decades of policy decisions that have shaped who gets access to affordable medicine-and who doesn’t.
Why Some Countries Have Hundreds of Generic Options, While Others Have None
In the United States, nearly 90% of prescriptions filled are for generic drugs. That’s higher than most other industrialized nations. But here’s the twist: even with that volume, the U.S. still pays the highest prices in the world for those generics. Why? Because competition doesn’t always mean lower prices. In the U.S., a drug like metformin might have 20 manufacturers making it, but the price hasn’t dropped much in years. Instead, you get what experts call “phantom competition”-lots of companies on paper, but only a few actually selling at low prices. Some manufacturers quietly exit the market, leaving others to raise prices without fear of losing customers. Compare that to the United Kingdom. They have a system where pharmacists automatically substitute brand-name drugs with generics unless the doctor says no. That’s called mandatory substitution. As a result, 83% of prescriptions are filled with generics-and prices are among the lowest globally. The government negotiates bulk prices, and manufacturers compete on volume, not profit margins. In Germany and the Netherlands, similar policies drive generic use above 70%. But in countries like Switzerland and Italy, generics barely crack 20%. Why? Because doctors and patients still trust the brand name. Insurance systems reimburse brand-name drugs at the same rate as generics, so there’s no financial incentive to switch. In Japan, patients often pay out-of-pocket for prescriptions, and many assume cheaper means lower quality. Cultural trust in pharmaceutical brands runs deep-and it’s harder to change than a law.The Manufacturing Divide: India, China, and the Hidden Supply Chain
Most of the generic pills you take didn’t come from a U.S. or European lab. They came from India or China. India alone produces about 20% of the world’s generic drugs-and 40% of the ones used in the U.S. It’s no accident. India has been building its generic industry since the 1970s, using legal loopholes to copy patented drugs before they expired. Today, it has over 750 FDA-approved manufacturing facilities. That’s more than any other country. But here’s what most people don’t realize: not all Indian-made generics are created equal. A 2023 study from Ohio State University found that generic drugs made in India had a 54% higher rate of severe adverse events-including hospitalizations and deaths-compared to identical drugs made in the U.S. The difference? Manufacturing standards. In the U.S., the FDA does unannounced inspections. In India, inspections are often scheduled in advance. That gives factories time to clean up, fix issues, and hide problems. It’s not that all Indian drugs are unsafe. But the risk is real-and it’s unevenly distributed. China is catching up fast. Its number of FDA-approved facilities jumped from 12 in 2010 to 187 in 2023. But quality concerns follow them too. During the pandemic, when India restricted exports of key active ingredients, the U.S. saw shortages of antibiotics and blood pressure meds. China stepped in-but not all of their products met U.S. standards. The FDA rejected over 100 manufacturing sites in China and India in 2023 alone for failing inspections.Price Differences That Make No Sense
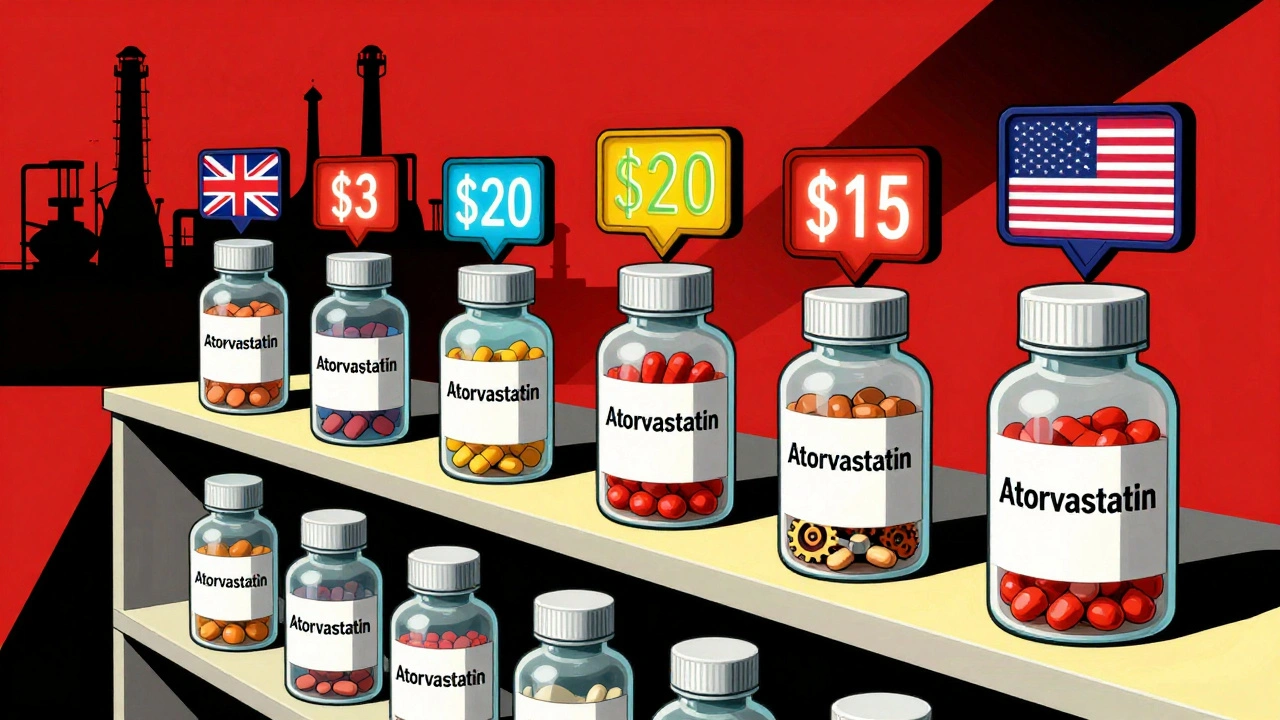
Let’s say you need a generic version of atorvastatin (Lipitor). In the U.S., a 30-day supply might cost $15. In Canada, it’s $8. In the U.K., it’s $3. In Switzerland? Over $20. Same pill. Same active ingredient. Same packaging. But the price? More than six times higher. This isn’t about production cost. It’s about pricing power. In countries with strong public health systems-like the U.K. or Germany-the government sets prices. In the U.S., prices are set by drug manufacturers, pharmacy benefit managers, and insurers in a system with almost no price controls. In Switzerland, insurers pay high prices because they’re legally required to cover brand-name drugs unless generics are proven to be “equivalent”-a bar that’s hard to meet. Even within Europe, the differences are stark. In Italy, generic adoption is below 20%. In the Netherlands, it’s over 70%. Why? Because Dutch pharmacists are paid a bonus for dispensing generics. Italian doctors aren’t. Simple as that. And then there’s the paradox: the U.S. uses the most generics, but pays the most. Why? Because the market is fragmented. There’s no central buyer. No single agency negotiating for 330 million people. Instead, you’ve got hundreds of insurers, each making their own deals. Some get good prices. Others pay full freight. And when a single manufacturer controls 80% of a generic market-like what happened with doxycycline in 2014-prices can spike overnight.
How Patients Get Caught in the Middle
I’ve heard from patients who travel to Canada for their meds. One woman from Ohio told me she fills her levothyroxine prescription in Toronto because it’s 70% cheaper. But when she switched from her U.S.-made version to the Canadian one, she started having heart palpitations. Turns out, the Canadian version used a different filler. Not the active ingredient-just the inactive ones. But for someone with thyroid disease, even tiny changes in absorption can cause real symptoms. Another patient in Florida found his generic metformin at a Mexican pharmacy for $4. He felt fine for months-until he started having nausea and dizziness. His doctor ran tests and found his blood sugar was all over the place. The Mexican version? It didn’t contain enough of the active ingredient. The FDA later flagged that batch as substandard. Even within the U.S., switching generics can be risky. A patient might be on one generic version of sertraline for years. Then, their pharmacy switches to a cheaper one from a different manufacturer. The patient doesn’t know. The doctor doesn’t know. But the patient starts feeling anxious, fatigued, or depressed. Why? Because even though bioequivalence standards say the drug must be within 80-125% of the brand’s absorption rate, that’s still a huge range. Two generics can be equally “approved” but behave very differently in the body.Why Global Harmonization Is So Hard
You’d think the solution is simple: standardize rules. Make every country use the same testing standards, the same inspection protocols, the same pricing rules. But that’s where politics gets in the way. The European Medicines Agency (EMA) and the U.S. FDA have similar bioequivalence rules-but they don’t accept each other’s data. A generic approved in the U.S. still needs a full review in Germany. That adds 18-24 months to market entry. Meanwhile, India’s regulators don’t require the same level of clinical data as the FDA. So a drug that’s approved in India might never make it to the U.S. market. The WHO tried to fix this with its 2024 Global Benchmarking Tool, pushing countries to adopt consistent quality standards. But enforcement? That’s up to each country. And many don’t have the resources-or the political will-to enforce them. Even the U.S. Inflation Reduction Act of 2022, which promised to speed up generic reviews by 30%, is still slow. The FDA is hiring more inspectors, but foreign facilities are growing faster than the agency can keep up.
What This Means for You
If you’re taking generic drugs, here’s what you need to know:- Don’t assume all generics are the same-even if they have the same name.
- If you notice new side effects after switching pharmacies, talk to your doctor. It might be a different manufacturer.
- Buying from international online pharmacies can save money-but it’s risky. Only use verified sites like PharmacyChecker-approved vendors.
- Ask your pharmacist: “Which manufacturer makes this generic?” Write it down. If you switch, ask if it’s the same one.
- For critical meds-thyroid, epilepsy, blood thinners-stick with the same brand or generic version if it works for you.
What’s Next?
The next big shift won’t be in small-molecule generics. It’ll be in biosimilars-copies of expensive biologic drugs like Humira or Enbrel. These are harder to make, harder to approve, and harder to substitute. But if they catch on, they could save the U.S. healthcare system over $100 billion by 2030. Until then, the global system stays broken. Some people get life-saving drugs for pennies. Others pay hundreds for the same thing. The problem isn’t that generics don’t work. It’s that the system designed to make them affordable is still stuck in national silos-with no real global oversight. The truth? Generic drugs are one of the greatest public health achievements of the last 50 years. But their promise can’t be fulfilled unless we fix how they’re made, priced, and trusted across borders.Why are generic drugs cheaper than brand-name drugs?
Generic drugs are cheaper because they don’t need to repeat expensive clinical trials. Once a brand-name drug’s patent expires, other companies can copy the active ingredient and prove it works the same way using faster, cheaper tests. They also don’t spend money on advertising or marketing. That’s why a generic version of a drug like metformin can cost 90% less than the original brand.
Are generic drugs as safe and effective as brand-name drugs?
Yes, for most people, generics are just as safe and effective. Regulatory agencies like the FDA and EMA require generics to meet strict bioequivalence standards-they must deliver the same amount of active ingredient into the bloodstream at the same rate as the brand-name drug. But there’s a catch: the allowed range for absorption is wide (80-125%), so two different generics of the same drug can behave differently in your body. This matters most for drugs with narrow therapeutic windows, like warfarin or levothyroxine.
Why do some countries have more generic options than others?
It depends on the country’s laws and healthcare system. Countries with mandatory generic substitution-like the U.K. and Netherlands-have high generic use because pharmacists are required to switch unless the doctor says no. In places like Switzerland and Italy, doctors and patients prefer brand names, and insurance pays the same for both, so there’s little incentive to switch. Also, countries with strong price controls, like Germany, attract more manufacturers because they can sell in bulk at low margins.
Is it safe to buy generic drugs from other countries online?
It can be risky. Many online pharmacies sell counterfeit or substandard drugs. Even legitimate ones might sell versions made in factories that don’t meet U.S. or European standards. The FDA doesn’t regulate foreign online pharmacies. If you buy from abroad, use only verified sites like those approved by PharmacyChecker. Never buy from sites that don’t require a prescription or that offer “miracle” discounts.
Why do generic drug prices sometimes spike suddenly?
Price spikes happen when there’s little competition. If only one or two companies make a generic drug and one of them shuts down or faces a shortage, the remaining company can raise prices dramatically. This happened with doxycycline in 2014 and cyclophosphamide in 2018. It’s called a “market failure”-not because the drug is bad, but because the system broke down. The FDA tracks these shortages, and in 2023, 68% of the 147 generic shortages were due to manufacturing issues, mostly in India and China.
Can I ask my pharmacist to keep giving me the same generic manufacturer?
Yes, you can. In most U.S. states, pharmacists are allowed to substitute generics unless the prescription says “dispense as written.” But you can ask your pharmacist to stick with the same manufacturer. Some pharmacies will even note your preference in your profile. If you notice side effects after switching, tell your doctor and pharmacist right away. Your health matters more than cost savings.
Rebecca M.
December 2, 2025 AT 19:38So let me get this straight-we pay $15 for a pill that costs 3 cents to make, and the only thing stopping us from fixing this is... capitalism? 😂
Meanwhile, my grandma in Ohio is driving to Canada just to not go broke. I swear, if this was a Netflix show, it’d be called ‘Pharmageddon: The Generic Wars’.
And don’t even get me started on the ‘bioequivalent’ BS. My thyroid meds switched manufacturers last month and I felt like a zombie who lost her Wi-Fi. Same pill? Nah. Same *experience*? Absolutely not.
Who’s really winning here? The shareholders. Not us. Not the patients. Just the suits in boardrooms who think ‘80-125% absorption range’ is a feature, not a flaw.
Someone please explain to me why we’re still letting this happen. I’m not mad. I’m just disappointed. And slightly nauseous.
Also, why does India get blamed for everything? We’re the ones buying the damn pills. We’re the ones demanding cheap. We’re the ones outsourcing the manufacturing and then acting shocked when the quality’s iffy. Hypocrisy is a full-time job in this country.
And yet… we still trust the FDA? 😅
Anyway. I’m off to buy my next prescription from a guy in a van parked outside a Walmart. He says it’s ‘FDA-approved’… but he also said his cousin invented the iPhone. So… yeah.
TL;DR: We’re all just rats in a lab, and the cheese is our last paycheck.
Lynn Steiner
December 3, 2025 AT 15:23Ugh. I hate this so much. 😭
My dad died because he couldn’t afford his blood thinner. He switched generics. Didn’t know. Died in his sleep.
They say it’s ‘bioequivalent’. But my dad’s body didn’t know that. His heart didn’t care about FDA ranges.
And now we’re talking about India and China like they’re the villains? No. The villain is a system that lets people die so shareholders can get bonuses.
USA: Where you can buy a $10,000 pill for your dog but your mom can’t get her diabetes meds.
Stop pretending this is about ‘quality’. It’s about greed. Plain and simple.
And if you think ‘buying from Canada’ is the answer… you’re just delaying the inevitable.
Someone needs to burn this whole system down. 💥
Alicia Marks
December 5, 2025 AT 02:57You’re not alone. Many people are struggling with this. But there are steps you can take to protect yourself.
Ask your pharmacist which manufacturer made your pill.
Write it down. Stick with it if it works.
And if you feel off after a switch-tell your doctor immediately.
You have power in this system. Use it.
Small actions add up. You’re not powerless. 💪
Paul Keller
December 6, 2025 AT 06:40While the anecdotal evidence presented is compelling, it is imperative to contextualize the broader structural dynamics at play. The United States, as a market-driven healthcare ecosystem, lacks the centralized price negotiation mechanisms inherent in single-payer systems. Consequently, the phenomenon of price disparities is not anomalous but rather an emergent property of decentralized procurement and the absence of antitrust enforcement in the generic pharmaceutical sector.
Furthermore, the regulatory divergence between the FDA and EMA, while inefficient, reflects differing risk tolerances and historical institutional trajectories. The 80-125% bioequivalence window, though broad, is statistically validated across millions of patient-years and remains the international standard for good reason.
That said, the recent spike in manufacturing-related shortages-particularly those traceable to non-compliant facilities in India and China-underscores a systemic vulnerability that demands coordinated international oversight, not merely consumer-level workarounds.
It is not merely a matter of cost, but of supply chain resilience. The U.S. has outsourced its pharmaceutical manufacturing capacity to the point of strategic dependency, and this requires policy recalibration, not just personal vigilance.
Perhaps the solution lies not in national isolationism, but in harmonized regulatory frameworks with enforceable quality benchmarks-modeled on WHO’s emerging tools, but with teeth.
And yes, patients should absolutely track their generic manufacturers. But systemic change requires legislative action, not just pharmacy-level awareness.
It is time we stop treating this as a personal burden and recognize it as a public health imperative.
Shannara Jenkins
December 7, 2025 AT 02:01Thank you for writing this. It’s so easy to feel powerless when you’re just trying to survive and stay healthy.
I used to switch generics without a second thought-until my anxiety went from ‘manageable’ to ‘I can’t leave the house’.
Turns out, the new version had a different filler. Tiny change. Huge impact.
Now I always ask my pharmacist: ‘Is this the same one?’
They roll their eyes sometimes, but they do it.
And guess what? My doctor started keeping notes in my chart too.
It’s not perfect, but it’s a start.
If you’re on meds that affect your brain or heart-don’t just accept the switch. Speak up. You’re worth it. ❤️
मनोज कुमार
December 7, 2025 AT 23:42Joel Deang
December 8, 2025 AT 16:09omg i just realized my metformin switched again 😭
i was like why am i so tired and hungry all the time
then i checked the bottle and it was a totally diff company
and now i’m scared to even take it
also i bought some from mexico last year and it was like $3 but my blood sugar was all over the place
why does this have to be so hard??
also can we talk about how the FDA is literally behind by 10 years??
imagine if your phone got updates slower than this
😭🙏
Roger Leiton
December 9, 2025 AT 13:42Okay but have we considered that maybe the real issue isn’t the drugs-it’s that we’ve turned healthcare into a commodity?
Like… we accept that a life-saving pill should cost more than a new iPhone?
And yet we’re shocked when people start buying meds from vans in parking lots?
The system isn’t broken. It’s working exactly as designed.
And the worst part? We’re all complicit.
We don’t vote on this. We don’t protest. We just Google ‘how to get cheap meds’ and hope for the best.
Meanwhile, the people who make these drugs? They’re working 12-hour shifts in factories with no air conditioning, just to keep the price low.
So who’s really the villain here?
Just… food for thought. 🤔
Laura Baur
December 10, 2025 AT 16:04Let’s be brutally honest: the entire global generic drug infrastructure is a moral catastrophe disguised as a public health achievement. The fact that we are even having this conversation-that people are crossing borders to buy pills their own government refuses to subsidize-is not a quirk of economics. It is a failure of civilization.
And yet, we continue to elevate the FDA as some kind of infallible oracle while ignoring that it inspects Indian factories on schedule, not randomly, because it lacks the funding, the will, or the political courage to do otherwise. This is not oversight. This is theater.
Meanwhile, patients are being turned into pharmacists, detectives, and importers-all because the system refuses to treat medicine as a human right. You want to know why people die? It’s not because of fillers. It’s because we’ve normalized the idea that your life is worth what you can afford.
And don’t even get me started on the cultural bias in countries like Japan and Switzerland-where people equate price with purity. That’s not trust. That’s ignorance masquerading as tradition.
What we need is not more advice on ‘asking your pharmacist’-we need a global treaty on pharmaceutical equity. We need to abolish patent monopolies on life-saving generics. We need to treat medicine like clean water-not a luxury item auctioned off to the highest bidder.
Until then, we are all just spectators at a slow-motion massacre.
And you? You’re just buying time.
Jack Dao
December 10, 2025 AT 20:29Let’s be real-this whole thing is a joke. You people act like you’re shocked that corporations are greedy. Newsflash: they’re supposed to be. That’s their job.
And you think India and China are the problem? Please. They’re the only reason you’re not paying $200 for metformin.
Stop pretending you care about ‘quality’ when your only concern is price. You want cheap? You get what you pay for. End of story.
And don’t even get me started on the ‘I switched generics and felt weird’ crowd. That’s not a drug issue-that’s a placebo issue. You’re not special.
Maybe if you stopped complaining and just took the damn pill, the system wouldn’t be so broken.
Also, buying from Mexico? You’re literally playing Russian roulette with your life. And you wonder why we have shortages?
Grow up. This isn’t a Netflix doc. It’s capitalism. Deal with it.
And if you’re so worried, go work for the FDA. Maybe you can fix it yourself. 😏