Drug Ingredient Checker
Check if your medications contain duplicate active ingredients and stay within safe daily limits.
Your Medication List
No medications added yet
Safe Usage
You're within safe dosage limits.
Warning: This tool only checks common OTC ingredients. Always consult your pharmacist or doctor for medication interactions. Maximum daily limit for acetaminophen is 4000 mg. For other drugs, consult your healthcare provider.
Every time you pick up a bottle of pain reliever, cold medicine, or even hand sanitizer, you’re holding a product regulated by the FDA - and the key to using it safely is right on the label. It’s not just a bunch of small print. It’s a standardized, science-backed guide called the OTC Drug Facts label. If you’ve ever taken too much acetaminophen, mixed medications that shouldn’t be combined, or wondered why a product says "do not use if you have high blood pressure," the answer is on that label. Yet most people skip it. And that’s where things go wrong.
Why the Drug Facts Label Exists
Before 1999, OTC drug labels were a mess. One brand listed ingredients in random order. Another hid warnings in tiny font. Some didn’t even say how much to take. The FDA stepped in because people were getting hurt - not from the medicine itself, but from using it wrong. A 2018 FDA study found that when labels weren’t standardized, only 29% of people could figure out the right dose. After the Drug Facts format rolled out, that number jumped to 76%. That’s not just a number. That’s fewer hospital visits, fewer overdoses, fewer mistakes. The rule is simple: every OTC product - whether it’s ibuprofen, antacid, sunscreen, or anti-cavity toothpaste - must use the same format. No exceptions. The goal? Make it easy to find the right info, fast, no matter what brand you buy.The 8 Sections of the Drug Facts Label (In Order)
The label isn’t random. It’s built like a checklist you can scan in seconds. Here’s what’s always there, in this exact order:- Drug Facts - The title. Always capitalized, always at the top.
- Active Ingredient(s) - The medicine part. This is the most important section. It tells you exactly what’s doing the work - and how much. For example: "Acetaminophen 325 mg". If a product has multiple active ingredients, they’re listed alphabetically. Never ignore this. It’s your first line of defense against doubling up on the same drug.
- Purpose(s) - What kind of drug is this? Is it a pain reliever? An antihistamine? A cough suppressant? This tells you the category. If you’re unsure why you’re taking something, this section explains it.
- Use(s) - What symptoms or conditions does it treat? "Relieves minor aches and pains" or "Reduces fever". If your symptom isn’t listed, don’t use it. This isn’t a cure-all. It’s targeted.
- Warnings - This is where the FDA forces manufacturers to be loud. Serious risks go in red boxes. If you have liver disease, asthma, or high blood pressure, you’ll see it here. "Do not use if..." means stop. "Ask a doctor before use if..." means get advice. "Stop use and ask a doctor if..." means stop now and call. These aren’t suggestions. They’re safety rules.
- Directions - How much? When? How often? This section gives exact instructions. "Adults: Take 2 tablets every 4 to 6 hours as needed. Do not exceed 8 tablets in 24 hours." If it says "for children under 12, consult a doctor," don’t guess. Call. Also, watch for units - teaspoons, milliliters, milligrams. Never use a kitchen spoon. Use the measuring cup or syringe that came with the product.
- Other Information - Storage tips ("Keep at room temperature") and sodium content (if it’s taken by mouth). Yes, some pain relievers have salt in them. If you’re on a low-sodium diet, this matters.
- Inactive Ingredients - These aren’t medicine, but they can still hurt you. Dyes, flavors, preservatives. If you’re allergic to sulfites, gluten, or red dye #40, this is where you find out. People skip this, then wonder why they got a rash or stomach upset.
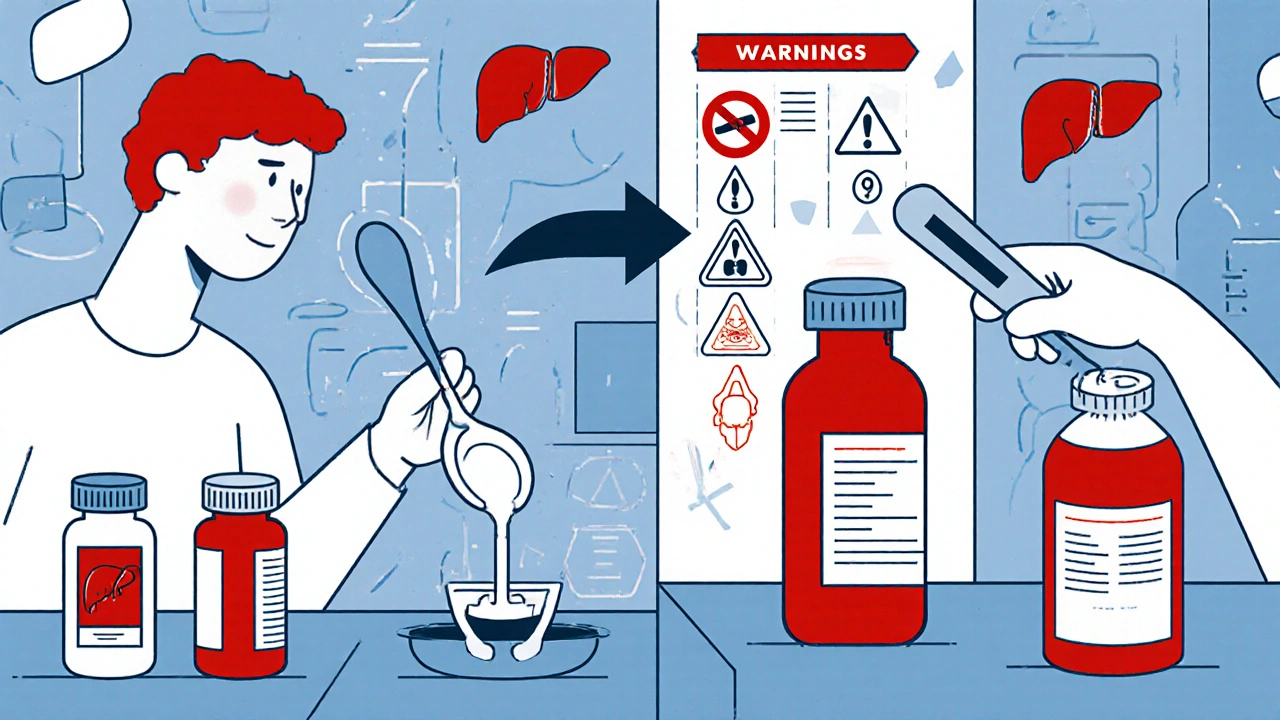
What You’re Probably Missing (And Why It Matters)
Most people look at the brand name and the dose. That’s it. But here’s what most miss:- Active ingredient overlap - Cold medicine, headache pills, and sleep aids often all contain acetaminophen. Taking two? You could hit 4,000 mg in a day - the max safe limit. Liver damage doesn’t wait. It sneaks up.
- "Ask a doctor" vs. "Stop use" - "Ask a doctor if you have high blood pressure" means you can still use it, but talk to someone first. "Stop use if you develop a rash" means stop immediately. One is a caution. The other is an emergency.
- Expiration dates - The label doesn’t always say "expires on." Sometimes it’s printed as a lot number. If you can’t read it, toss it. Expired medicine doesn’t work right - and some can break down into harmful chemicals.
- Children’s dosing - Never use adult dosing for kids. Even if the child is small. Weight-based dosing matters. If the label doesn’t list a dose for your child’s age or weight, don’t use it. Call your pediatrician.
Real-Life Mistakes (And How to Avoid Them)
A 2022 report from the Institute for Safe Medication Practices found that 41% of OTC medication errors happen because people didn’t check for duplicate ingredients. A man took a cold medicine with acetaminophen, then took extra Tylenol for a headache. He ended up in the ER with liver failure. He didn’t realize both had the same active ingredient. Another common error? Using a kitchen tablespoon for liquid medicine. A tablespoon is 15 mL. A dosing cup says 5 mL. That’s three times too much. That’s dangerous. And then there’s the "I’ll just take a little more if it’s not working" mindset. The label says "every 6 hours." Taking it every 4? That’s not being proactive. That’s risking overdose. The fix? Make a habit. Before you take any OTC drug, stop. Read the label. Ask yourself: "What’s in this? Does it match what I’m already taking? Am I in the right group to use this? Am I giving the right dose?" It takes 30 seconds. It could save your life.
What’s New? Digital Labels and QR Codes
The FDA is testing new ways to make labels clearer. Some products now have QR codes that link to video instructions, multilingual versions, or detailed warnings. But here’s the catch: the physical label still has to include all the Drug Facts info. The digital part is extra. Don’t rely on your phone to read the label. If you’re in a hurry, or your phone’s dead, the printed label must still work. Also, more products now combine drugs with cosmetics - like deodorants with antiperspirant or moisturizers with SPF. These have special labeling rules too. If it claims to treat or prevent a condition, it’s a drug. And it needs a Drug Facts label.What to Do If You’re Still Confused
If the label doesn’t make sense, don’t guess. Call your pharmacist. They’re trained to read these labels and spot interactions. You can also visit the FDA’s website for free guides on reading OTC labels. Or use the Poison Control hotline (1-800-222-1222) if you’re worried you’ve taken too much. The bottom line: OTC doesn’t mean "safe without thinking." It means "safe if you know what you’re doing." The Drug Facts label is your cheat sheet. Use it every time.Do all OTC products have a Drug Facts label?
Yes. By FDA law, every over-the-counter drug - including pain relievers, cold medicines, antacids, hand sanitizers, sunscreens, and anti-cavity toothpaste - must have a Drug Facts label. If it doesn’t, it’s either not a regulated drug or it’s being sold illegally.
Can I use an expired OTC medicine?
The FDA says most OTC medicines remain safe after their expiration date, but they may lose effectiveness. For things like allergy meds or pain relievers, a few months past expiry might be okay. But for epinephrine auto-injectors, insulin, or liquid antibiotics, never use expired products. If you’re unsure, throw it out. Safety isn’t worth the risk.
Why do some labels say "Keep out of reach of children" and others don’t?
All OTC drugs that can be harmful if swallowed must include this warning. If you don’t see it, the product is likely not classified as a drug - maybe it’s a cosmetic or dietary supplement. But don’t assume it’s safe. Always check the label type. If it says "Supplement Facts," it’s not regulated like a drug and may have unknown risks.
Is it safe to take OTC meds with prescription drugs?
Not always. Many OTC pain relievers, antacids, and cold medicines interact with blood thinners, antidepressants, or blood pressure meds. The Drug Facts label will warn you if there’s a known interaction. But if it doesn’t mention your prescription, ask your pharmacist. They have access to full interaction databases that go beyond the label.
What’s the difference between active and inactive ingredients?
Active ingredients are the medicine - the parts that treat your symptom. Inactive ingredients are everything else: fillers, dyes, flavors, preservatives. They don’t treat anything, but they can cause allergic reactions. If you’re allergic to red dye, corn starch, or sulfites, check the inactive list. Many people don’t realize their rash or stomach upset came from an inactive ingredient, not the drug itself.

jobin joshua
November 30, 2025 AT 05:38OMG this is so true!! 😱 I once took cold medicine + Tylenol and felt like my liver was gonna explode 🤯 Glad I read this before it was too late!!
Sachin Agnihotri
November 30, 2025 AT 16:49Wow, I never realized how much structure goes into these labels! I used to just glance at the brand and dose... now I actually read every line. It's wild how many people die from simple mistakes like this. Seriously, everyone should print this out and tape it to their medicine cabinet. 🙏
Diana Askew
December 2, 2025 AT 09:45Of course the FDA made this... but do you think Big Pharma even wants you to read it? They profit when you overdose. They profit when you mix drugs. They profit when you're confused. This is all a distraction. The real danger? The pills themselves. Always question the source. 🕵️♀️
King Property
December 4, 2025 AT 09:11You people are idiots. If you can't read a label, you shouldn't be allowed to own a pharmacy. I've been reading these since I was 12. The fact that you need a blog post to understand 'acetaminophen = liver killer' is terrifying. Also, stop using kitchen spoons. That's not a suggestion. That's a crime against science.
Yash Hemrajani
December 4, 2025 AT 11:03Oh wow, so the label actually has info? Who knew? 😏 I thought it was just decorative text between the logo and the barcode. Next time I take NyQuil, I'll try not to confuse it with my shampoo. Thanks for the laugh, OP.
Pawittar Singh
December 6, 2025 AT 00:36THIS IS LIFE-SAVING STUFF. 🙌 Seriously, share this with your grandma, your cousin who takes 5 different meds, your roommate who thinks 'a little extra won't hurt.' We're all just trying to survive. Let's not accidentally kill each other with ibuprofen. 💪❤️
Josh Evans
December 6, 2025 AT 16:57Agreed. I used to ignore the label too. Now I read it every time. Even for hand sanitizer. Who knew some had alcohol and others don't? Small stuff, but it adds up. Thanks for the reminder.
Allison Reed
December 7, 2025 AT 19:00Excellent breakdown. The structure of the Drug Facts label is one of the most effective public health interventions in modern history. Standardization saves lives. It’s not glamorous, but it works. This should be mandatory reading in high school health classes.
Jacob Keil
December 9, 2025 AT 18:06so like... if the label says 'do not use if you have high blood pressure' but you kinda feel fine... is it still bad? i mean... life is risk. we're all gonna die someday. maybe this is just the universe telling me to chill. 🤔
Rosy Wilkens
December 10, 2025 AT 13:23How can we trust anything the FDA says? They approved opioids. They approved aspartame. They approved vaccines with undisclosed ingredients. This 'Drug Facts' label is just another marketing tool to make you feel safe while they quietly poison you. Look at the fine print. Always. Always. Always.
Andrea Jones
December 12, 2025 AT 07:50Love this! And honestly? The QR code thing is genius. Imagine scanning it and getting a 30-second video in Spanish, or with sign language. But please-don’t ditch the printed label. Not everyone has a phone, or good eyes, or Wi-Fi. Keep it simple. Keep it clear. Keep it human.