Therapeutic Equivalence Code Lookup
Check if a generic drug is safe for substitution
Enter a drug name to see its Therapeutic Equivalence (TE) code according to the FDA's Orange Book. This will tell you if the generic version can safely substitute for the brand-name drug.
Therapeutic Equivalence Information
The Orange Book isn’t a book you read for fun-it’s the single most important reference pharmacists, doctors, and insurers use every day to decide which generic drugs can safely replace brand-name medications. Officially titled Approved Drug Products with Therapeutic Equivalence Evaluations, it’s published by the U.S. Food and Drug Administration (FDA) and updated every month. Since 1980, it’s been the backbone of America’s $1.67 trillion in generic drug savings over the last decade. If you’ve ever picked up a cheaper version of your prescription and wondered why it was allowed, the answer is in the Orange Book.
What Makes a Generic Drug Safe to Substitute?
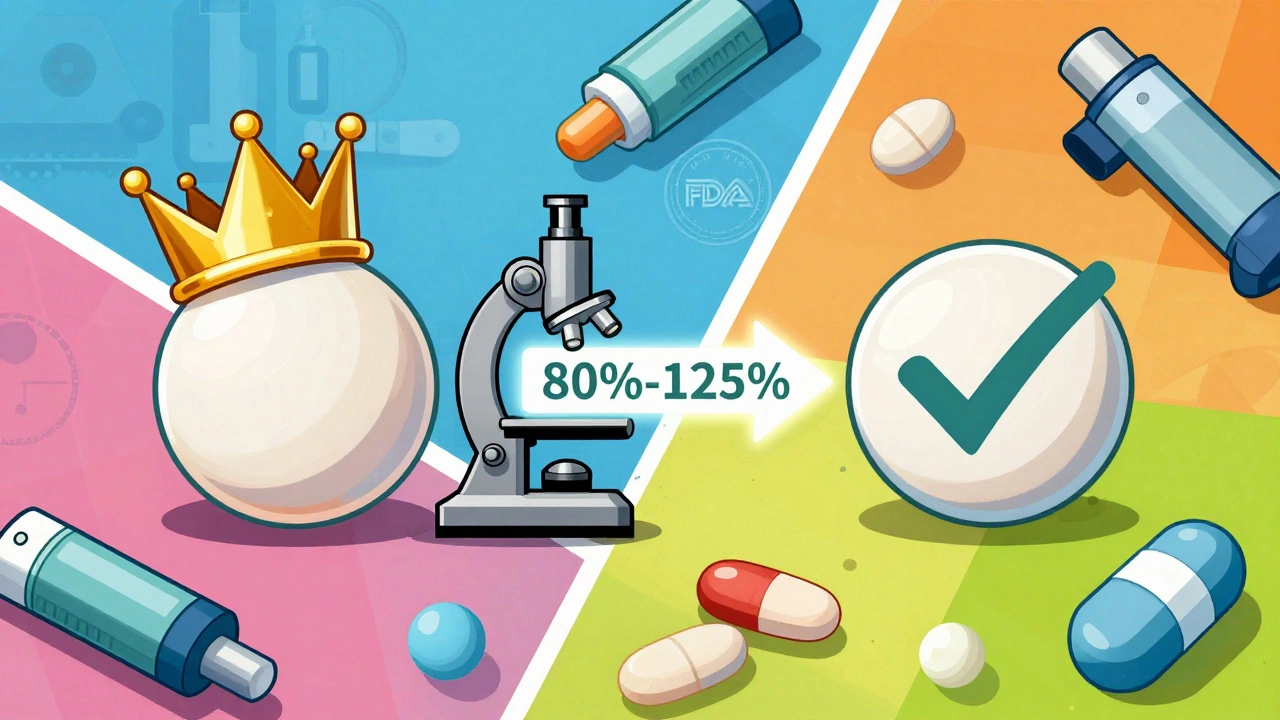
Not all generics are created equal. The FDA doesn’t just approve any pill that looks like the brand name. To be listed in the Orange Book as therapeutically equivalent, a generic drug must pass three strict tests: pharmaceutical equivalence, bioequivalence, and regulatory compliance.
Pharmaceutical equivalence means the generic has the exact same active ingredient, in the same strength, same dosage form (tablet, capsule, injection), and same route of administration (oral, topical, etc.) as the brand-name drug. It must also meet the same quality and purity standards set by the U.S. Pharmacopeia.
Bioequivalence is where things get technical but critical. It means your body absorbs and processes the generic drug at the same rate and to the same extent as the brand. If a generic drug hits your bloodstream too slowly or too quickly, it could be ineffective-or even dangerous. The FDA requires bioequivalence studies showing that the generic’s absorption falls within 80% to 125% of the brand’s levels. That’s a tight window, especially for drugs with a narrow therapeutic index like warfarin or levothyroxine.
Finally, the manufacturer must follow Current Good Manufacturing Practices (CGMP). This isn’t optional-it’s enforced by routine FDA inspections. A drug can’t be listed in the Orange Book unless the FDA is confident it’s made safely, consistently, and without contamination.
The TE Code System: Decoding the Letters
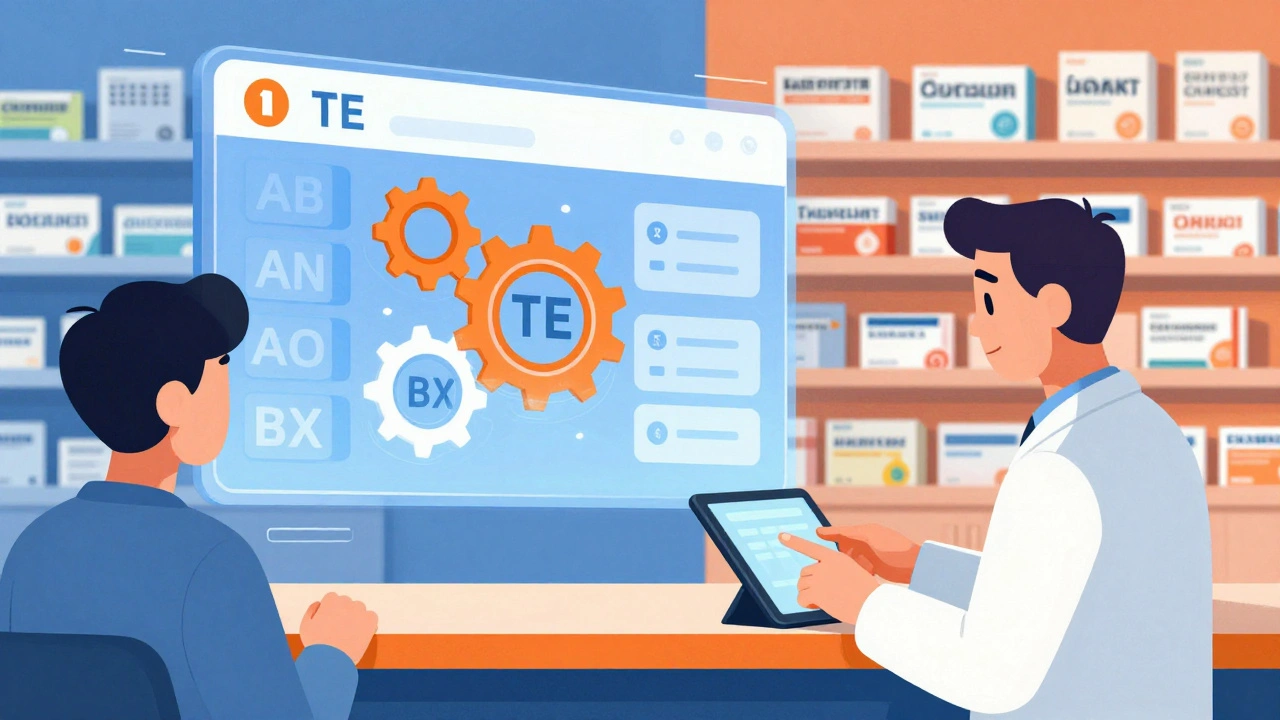
The Orange Book uses a simple but powerful coding system to tell you whether a generic can be substituted. These are called Therapeutic Equivalence (TE) codes, and they’re the first thing pharmacists check when filling a prescription.
All therapeutically equivalent drugs start with an A. The second letter tells you more:
- AB = Fully approved, no known bioequivalence issues. These are the safest substitutes.
- AN = Approved for nasal inhalation products. These are complex delivery systems, but still considered equivalent.
- AO = Approved for oral inhalation products, like asthma inhalers.
- AP = Approved for parenteral (injectable) products.
Drugs that are not considered equivalent start with B:
- BC = Bioequivalence problems suspected but not confirmed.
- BD = Bioequivalence data is inadequate or conflicting.
- BX = The FDA has determined the product is not therapeutically equivalent. These should never be substituted without a doctor’s approval.
For example, if your prescription says Simvastatin 40 mg and the pharmacy has an AB-coded generic, you can get it without a new prescription. But if the generic is coded BX, the pharmacist must contact your doctor before switching.
Who Uses the Orange Book-and Why?
The Orange Book isn’t just for pharmacists. It’s used by insurance companies, pharmacy benefit managers (PBMs), state health agencies, and even hospitals to control costs and ensure patient safety.
Most insurance plans require you to try the generic version first-unless your doctor writes "dispense as written" or "no substitution." That’s because generics cost 80-90% less than brand-name drugs. In 2023, 90.7% of all prescriptions filled in the U.S. were for generics, but they made up only 22.8% of total drug spending. That’s billions saved every year.
Pharmacists rely on the Orange Book daily. A 2023 survey by Pharmacy Times found that 92% of pharmacists consider it essential. But here’s the problem: 67% said the TE code system is moderately to extremely difficult to interpret. Many get confused between AB, AN, and AO codes-especially for inhalers and injectables. One Walgreens study found that misreading TE codes led to $1.2 million in rejected insurance claims in just one quarter.
Some pharmacies, like CVS Health, have fixed this with automated systems. Since launching their TE code checker in 2021, they’ve cut substitution errors by 63% and saved $47 million annually. That’s not just efficiency-it’s patient safety.
What’s Not in the Orange Book?
The Orange Book doesn’t cover everything. It only includes drugs approved under the New Drug Application (NDA) or Abbreviated New Drug Application (ANDA) pathways. That means some older drugs are left out.
For example, drugs approved before 1938-like phenobarbital-or those still under review by the Drug Efficacy Study Implementation (DESI) program-like Librax-are not listed. These aren’t necessarily unsafe, but they never went through the modern FDA approval process.
Also excluded are combination products where the device (like an inhaler or auto-injector) is considered part of the drug. The FDA evaluates these on a case-by-case basis. If the generic device works the same way and delivers the drug identically, it can get an A code. But if the device differs significantly, the FDA may require extra testing. That’s why some inhaler generics carry BX codes-they’re chemically identical, but the delivery mechanism might not be.
Complex Drugs and the Future of Substitution
Not all drugs are easy to copy. Inhalers, topical creams, injectables, and transdermal patches are considered "complex generics." Their effectiveness depends not just on the chemical, but on how the drug is delivered.
In 2022, the FDA released new guidance to clarify how to evaluate these products. The message was clear: the generic must produce the same clinical effect and safety profile as the brand. That doesn’t mean the device has to be identical-it just has to work the same way.
But challenges remain. For example, there are over 30 different generic versions of the asthma inhaler albuterol. Some have AB codes. Others are BX. Why? Because bioequivalence testing for inhalers is harder. The particle size, spray pattern, and lung deposition matter. A small difference in how the drug is delivered can mean the difference between relief and a hospital visit.
The FDA is working on a new digital version of the Orange Book, set to launch in Q2 2024. It will include application numbers, manufacturer names, and drug strengths all in one searchable database. That should make it easier for pharmacists to verify substitutions quickly.
What You Should Know as a Patient
If you’re on a generic medication, you’re probably saving money. But you should still know what you’re taking.
- Check your prescription label. Does it say "generic"? That’s fine.
- Ask your pharmacist: "Is this generic approved as therapeutically equivalent?" They can check the Orange Book in seconds.
- If you notice a change in how the drug works-more side effects, less effectiveness-tell your doctor. It could be a different generic.
- Don’t assume all generics are interchangeable. Even two AB-coded versions of the same drug can have different inactive ingredients, which might affect you if you have allergies.
The Orange Book is designed to protect you. It’s not a marketing tool. It’s a scientific standard. When a drug has an AB code, you can trust it.
Training and Resources
Pharmacists spend an average of 12.7 minutes per complex prescription verifying therapeutic equivalence using the Orange Book. That’s time they could spend counseling patients.
The FDA offers free online training modules. The National Community Pharmacists Association (NCPA) runs a 4-hour certification course on TE codes-over 8,000 pharmacists took it in 2022. But only 41% of community pharmacists say they feel "very confident" using the Orange Book without help.
Patients don’t need to be experts. But knowing the basics-like what an AB code means-can help you ask better questions and avoid errors.
What does "AB" mean in the Orange Book?
"AB" means the generic drug is therapeutically equivalent to the brand-name drug. It has the same active ingredient, dosage form, and bioavailability. It’s approved for substitution without a doctor’s permission.
Can I always switch to a generic drug?
Not always. If your prescription says "dispense as written" or "no substitution," your doctor wants the brand. Also, if the generic has a "BX" code, it’s not approved for substitution. Always check with your pharmacist before switching.
Why do some generic drugs cost more than others?
Even if two generics have the same TE code, prices can vary because of manufacturing costs, supply chain issues, or market competition. A lower price doesn’t mean lower quality-both are approved as equivalent. But if you’re switching between generics and notice side effects, tell your doctor.
Are all generic drugs tested by the FDA?
Yes. Every generic drug must be approved by the FDA before it can be sold. The FDA reviews bioequivalence data, manufacturing practices, and labeling. The Orange Book only lists drugs that pass these standards.
How often is the Orange Book updated?
The Orange Book is updated every month. New generics are added, TE codes are changed, and withdrawn drugs are removed. Pharmacists and insurers rely on these updates to make accurate substitution decisions.
What’s Next for the Orange Book?
The future of generic substitution depends on keeping the Orange Book accurate and accessible. The FDA’s new digital platform will make it easier to search for TE codes, check manufacturers, and verify drug strengths-all in one place.
As more complex drugs enter the market-like biosimilars and personalized therapies-the FDA will need to adapt. But the core principle remains: if a drug is approved as therapeutically equivalent, it’s safe to substitute. That’s not just policy-it’s public health.
For patients, that means lower costs. For the system, it means sustainability. And for the FDA, it means staying true to its mission: ensuring safe, effective, and affordable medicines for everyone.

Marvin Gordon
December 5, 2025 AT 17:01The Orange Book is one of those quiet heroes of modern medicine. Nobody talks about it, but if it disappeared tomorrow, half the prescriptions in this country would get stuck in limbo. It’s not glamorous, but it’s the reason your insulin costs $30 instead of $300. Respect.
Rupa DasGupta
December 7, 2025 AT 14:03OMG this is so true!! 🙌 I just got my generic levothyroxine switched again and I swear I felt like a zombie for two weeks. The AB code says it’s fine but my body knows better. Who even made these rules?? 😭
Mellissa Landrum
December 9, 2025 AT 06:14They say the FDA tests generics but I’ve seen the factories. Half of them are in India and China and the inspectors show up like once a year. This whole system is rigged. They want you on cheap pills so you don’t ask questions. BX codes? More like BX for ‘Buy Their Stock’.
Mark Ziegenbein
December 11, 2025 AT 02:40Let’s be honest-the Orange Book is a masterpiece of bureaucratic elegance. The TE coding system, while cryptic to the layperson, is a triumph of regulatory precision. It balances pharmaceutical science with economic pragmatism, and frankly, most people don’t deserve to understand it. The fact that it’s updated monthly shows an institutional commitment to accuracy that most industries would kill for. The 80-125% bioequivalence window? Not arbitrary. It’s derived from pharmacokinetic variance models validated across thousands of clinical trials. And yet, we let pharmacists with 12 minutes per script interpret it. That’s not efficiency. That’s gambling with physiology.
Jennifer Patrician
December 12, 2025 AT 18:37Oh please. The FDA is just a puppet of Big Pharma. They let generics in so the drug companies can raise brand prices even higher. And don’t get me started on how they classify inhalers. One puff different and you’re choking. That’s not science-that’s corporate sabotage. They don’t care if you die as long as the stock price goes up.
Lynette Myles
December 12, 2025 AT 22:23AB means safe to swap. BX means don’t. Simple. Stop overcomplicating it.
Juliet Morgan
December 13, 2025 AT 22:14I used to be scared to switch generics until I learned what AB meant. Now I ask my pharmacist every time. It’s not about being paranoid-it’s about being smart. If you feel different after a switch, speak up. Your body isn’t wrong.
Annie Grajewski
December 14, 2025 AT 00:03So wait… the FDA says two generics are the same but one costs 3x more? Sounds like a scam. I bet the expensive one has glitter in it or something. Also why does the book have ‘AO’? Is that like ‘Aqua Oxygen’? I’m confused and also kinda mad now.
Mark Curry
December 15, 2025 AT 08:13It’s funny how we trust a pill more than a person. We don’t question the science behind the Orange Book, but we’ll argue about whether a movie is good. Maybe we need more of this kind of quiet, boring reliability in our lives.
Jimmy Jude
December 16, 2025 AT 01:20The Orange Book is the last bastion of truth in a world run by algorithms and profit margins. It doesn’t care about your political views, your social media clout, or your influencer endorsements. It just says: does this work the same? If yes, fine. If no, stay out. That’s integrity. And it’s dying.
ashlie perry
December 17, 2025 AT 22:29They’re tracking your meds through the Orange Book to build a profile. Next they’ll use it to raise your insurance rates if you use too many generics. They already know you’re on simvastatin. They always know.