When you fill a prescription for a brand-name drug like Concerta or Celebrex, you might be surprised to get a pill that looks almost identical but has a different label. That’s not a mistake. That’s an authorized generic.
Authorized generics are not a new kind of drug. They’re the exact same medication as the brand-name version-same active ingredient, same inactive ingredients, same shape, same strength, same manufacturer. The only difference? No brand name on the box.
Think of it like buying a soda. The brand-name version has the red can with the classic logo. The authorized generic is the same soda, same recipe, same factory, same ingredients-but it’s in a plain can with no logo. You’re getting the exact same product, just without the brand name attached.
How Authorized Generics Are Made
Most generic drugs are made by other companies after the brand-name patent expires. Those companies have to prove their version works the same way through a process called the Abbreviated New Drug Application (ANDA). That takes time. They test bioequivalence, run stability studies, and wait for FDA approval.
Authorized generics skip all that.
The brand-name company itself-or one of its subsidiaries-makes the authorized generic. Because they’re using the exact same formula, equipment, and factory as the original brand, they don’t need to re-prove anything. They just notify the FDA and start selling it under a different label.
This means authorized generics are manufactured in the same facility, on the same production line, with the same quality controls as the brand-name version. There’s no variation in how it’s made. No guesswork. No risk of differences in absorption or effectiveness.
Why They Exist
Why would a drug company make its own generic?
It sounds odd. But it’s a smart business move.
When a brand-name drug’s patent expires, other companies can legally make and sell generic versions. The brand company loses market share fast. Prices drop. Profits shrink.
By launching an authorized generic, the original manufacturer can keep selling their drug-just under a cheaper label. They capture the patients who want a lower price but still trust the brand. Meanwhile, they slow down the rush to other generic competitors.
Research from Health Affairs in 2022 found that between 2010 and 2019, there were 854 authorized generic launches. In 75% of cases, the brand company waited until after a traditional generic had already entered the market before launching their own version. That’s not an accident. It’s strategy.
They’re not trying to help patients save money. They’re trying to protect their own revenue.
How They’re Different From Regular Generics
Here’s where things get confusing.
Regular generics only need to match the brand-name drug’s active ingredient. They can have different fillers, coatings, colors, or shapes. Sometimes those differences cause side effects-like stomach upset or allergic reactions-for people sensitive to certain inactive ingredients.
Authorized generics don’t have that problem. They’re chemically identical in every way. Same active ingredient. Same inactive ingredients. Same manufacturing process.
Take Colcrys, the brand-name drug for gout. Its authorized generic, made by Prasco Laboratories, uses the exact same formulation. If you’ve ever had a reaction to the brand, you’ll likely have the same reaction to the authorized generic-because it’s the same thing.
Regular generics? They might use a different dye, a different binder, or a different release mechanism. That’s why some patients say, “My generic doesn’t work like the brand.” Sometimes, it’s not the active ingredient-it’s the filler.
Authorized generics fix that. They’re the closest thing to the brand you can get without paying the brand price.
Where You’ll Find Them
Authorized generics aren’t listed in the FDA’s Orange Book-the official list of approved generic drugs. That’s because they’re not approved as generics. They’re approved under the original brand’s New Drug Application (NDA).
So how do you know you’re getting one?
You usually won’t. Pharmacists often don’t even know unless they check the manufacturer’s label. The pill might look identical to the brand. The box might say “colchicine” instead of “Colcrys.”
Some common examples include:
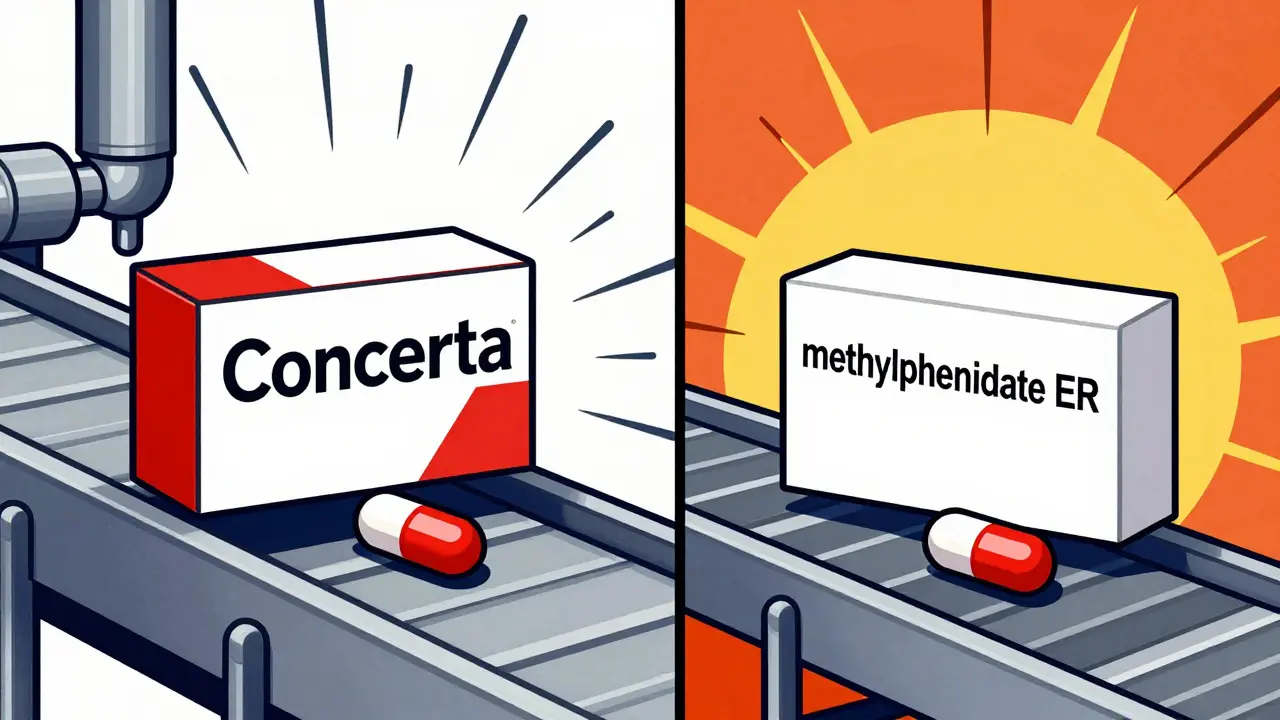
- Concerta → authorized generic by Watson/Actavis (methylphenidate ER)
- Celebrex → authorized generic by Greenstone Pharmaceuticals (celecoxib)
- Unithroid → authorized generic by Jerome Stevens Pharmaceuticals (levothyroxine)
- Colcrys → authorized generic by Prasco Laboratories (colchicine)
These aren’t rare. They’re common. If you’ve been on a brand-name drug for years and suddenly got a different-looking pill, it might be an authorized generic.
Price: Lower Than Brand, But Not Always Cheapest
Authorized generics are cheaper than the brand-name version-usually 15% to 25% lower.
But they’re not always the cheapest option.
Once multiple traditional generics enter the market, prices can drop even further. Sometimes by 80% or more.
So if you’re shopping for the lowest price, an authorized generic might not be the best deal. But if you’ve had bad reactions to other generics, or your doctor insists on consistency, it’s the safest alternative to the brand.
Some patients pay $120 for a 30-day supply of brand-name Celebrex. The authorized generic? $85. A traditional generic? $15.
It’s a trade-off: safety and consistency vs. lowest cost.
Patient Experience: Confusion and Trust
Many patients don’t even realize they’re taking an authorized generic. Pharmacists don’t always tell them. Insurance plans often don’t distinguish.
But when patients notice a change-different color, different imprint, different label-they get worried.
“Is this fake?”
“Did they switch me to a cheaper version?”
“Is it still as strong?”
These are valid concerns. The answer? Yes, it’s still the same drug. The FDA says so. The manufacturer says so. The pill has the same chemical fingerprint.
But the confusion is real. And it’s a problem.
Some patients stop taking their medication because they think it’s not the same. Others switch back to the brand, paying more than they need to.
Pharmacists need to explain it clearly: “This is made by the same company. Same factory. Same ingredients. Just no brand name.”

Regulatory Oversight
The FDA doesn’t approve authorized generics the way it does regular generics. There’s no ANDA. No bioequivalence testing.
Instead, the brand manufacturer files a simple notification. The FDA reviews it for accuracy, then allows it to be sold.
This means authorized generics are legally and scientifically identical to the brand-but they’re not tracked the same way. You won’t find them in the Orange Book. You won’t see them in most generic drug databases.
The FDA does maintain a public list of authorized generics, last updated in October 2025. But it’s not widely used or easily searchable. Most prescribers and pharmacists rely on manufacturer announcements or pharmacy systems to know when they’re available.
What This Means for You
If you’re on a brand-name drug and you’re paying a lot for it:
- Ask your pharmacist: “Is there an authorized generic for this?”
- Ask your doctor: “Can I switch to the authorized generic?”
- Check the pill’s imprint and manufacturer code. If it matches the brand, it’s likely the same thing.
If you’ve had issues with other generics-stomach pain, dizziness, inconsistent effects-an authorized generic might be your best option. No need to risk another trial-and-error switch.
If you’re cost-sensitive and don’t care about the filler ingredients, go for the cheapest traditional generic. But if you value consistency, safety, and peace of mind, the authorized generic is the smart middle ground.
It’s not a trick. It’s not a loophole. It’s a product made by the brand company, for people who want the brand-but without the brand price.
Future Outlook
Authorized generics aren’t going away. In fact, they’re likely to grow.
As more drugs lose patent protection, brand manufacturers will keep using them as a way to stay in the game. They’re a bridge between monopoly pricing and full generic competition.
Some experts worry they’re designed to delay true competition. Others see them as a practical way to give patients immediate access to lower-cost versions of drugs they trust.
Either way, you’ll see more of them. And knowing what they are-before you get confused-could save you money and stress.
Are authorized generics as safe as brand-name drugs?
Yes. Authorized generics are made by the same manufacturer, in the same facility, with the exact same ingredients and quality controls as the brand-name drug. The FDA considers them identical. The only difference is the label. If you’ve never had a problem with the brand, you won’t have one with the authorized generic.
Why aren’t authorized generics listed in the FDA’s Orange Book?
Because they’re not approved as generics. They’re marketed under the original brand’s New Drug Application (NDA). The Orange Book only lists drugs that went through the Abbreviated New Drug Application (ANDA) process. Authorized generics bypass that step, so they don’t appear there-even though they’re identical to the brand.
Can I switch from a brand-name drug to an authorized generic without my doctor’s approval?
In most cases, yes. Pharmacists can substitute authorized generics just like they do with regular generics. But if your prescription says “do not substitute,” you’ll need to ask your doctor to update it. Some insurers also require prior authorization for authorized generics, so check with your pharmacy.
Do authorized generics cost less than regular generics?
Usually not. Authorized generics are priced between the brand-name version and the cheapest traditional generic. They’re cheaper than the brand, but often more expensive than generics from multiple manufacturers. Their value isn’t in the lowest price-it’s in the identical formulation.
How do I know if I’m getting an authorized generic?
Check the manufacturer name on the bottle. If it’s the same company that makes the brand-name drug (e.g., Janssen for Risperdal, or Teva for Copaxone), it’s likely an authorized generic. You can also ask your pharmacist directly. Some pharmacies list it on the receipt. Online tools like GoodRx sometimes flag authorized generics in their pricing comparisons.
If you’re on a long-term medication and you’ve noticed changes in how you feel after switching pills, ask about an authorized generic. It might be the missing piece you didn’t know you needed.
Dennis Santarinala
February 16, 2026 AT 12:22