SEARCH
Drug Equivalence: What Makes Generics and Brand Drugs Interchangeable
When you hear drug equivalence, the scientific and regulatory standard that confirms two medications have the same clinical effect and safety profile. Also known as therapeutic equivalence, it's the reason your pharmacist can swap your brand-name pill for a cheaper version without asking your doctor. This isn’t just marketing—it’s science backed by the FDA, and it’s saving patients billions every year. If you’ve ever been told your prescription is now generic, you’re seeing drug equivalence in action.
Not all generics are created equal, though. That’s where authorized generics, exact copies of brand-name drugs made by the same company under a different label. Also known as brand-identical generics, they’re identical in every way—same ingredients, same factory, same quality control. Then there are regular generics, which meet FDA standards but might differ in fillers or coating. Both are safe, but authorized generics remove any doubt. The Orange Book, the FDA’s official list of approved drug products with therapeutic equivalence evaluations. Also known as Therapeutic Equivalence Codes (TE codes), it’s the public database that tells pharmacists which drugs can be swapped without risk. Look up your medication there, and you’ll see a TE code like AB1 or BX—AB means it’s interchangeable, BX means it’s not.
Why does this matter? Because people still worry. They think, "If it’s cheaper, is it weaker?" The truth is, the FDA requires generics to deliver the same amount of active ingredient into your bloodstream within the same timeframe as the brand. No exceptions. Studies show no meaningful difference in outcomes for conditions like high blood pressure, depression, or diabetes. But confusion lingers—especially when you see different prices for the same generic across pharmacies. That’s where global drug prices, the wide variation in cost for the same medication across countries due to pricing policies, manufacturing, and insurance systems come in. A pill that costs $5 in India might cost $50 in Switzerland—not because it’s different, but because of how it’s sold.
And here’s the kicker: if you’re switching from a brand to a generic, you’re not just saving money—you’re helping the whole system. Generic drugs make up over 90% of prescriptions in the U.S., but they cost less than 20% of the total drug spending. That’s drug equivalence working the way it should. You get the same results. The system gets more efficient. Everyone wins.
But not every drug plays nice. Some need exact dosing—like thyroid meds or blood thinners—so your doctor might stick with the brand. Others, like statins or antidepressants, have tons of proven generic options. The key is knowing what’s interchangeable and what’s not. That’s where the posts below come in. You’ll find clear breakdowns of how the Orange Book works, why authorized generics are the safest swap, how to spot fake pills masquerading as real ones, and what lab tests to watch for when switching meds. No fluff. No jargon. Just what you need to know to make smart, safe choices with your prescriptions.
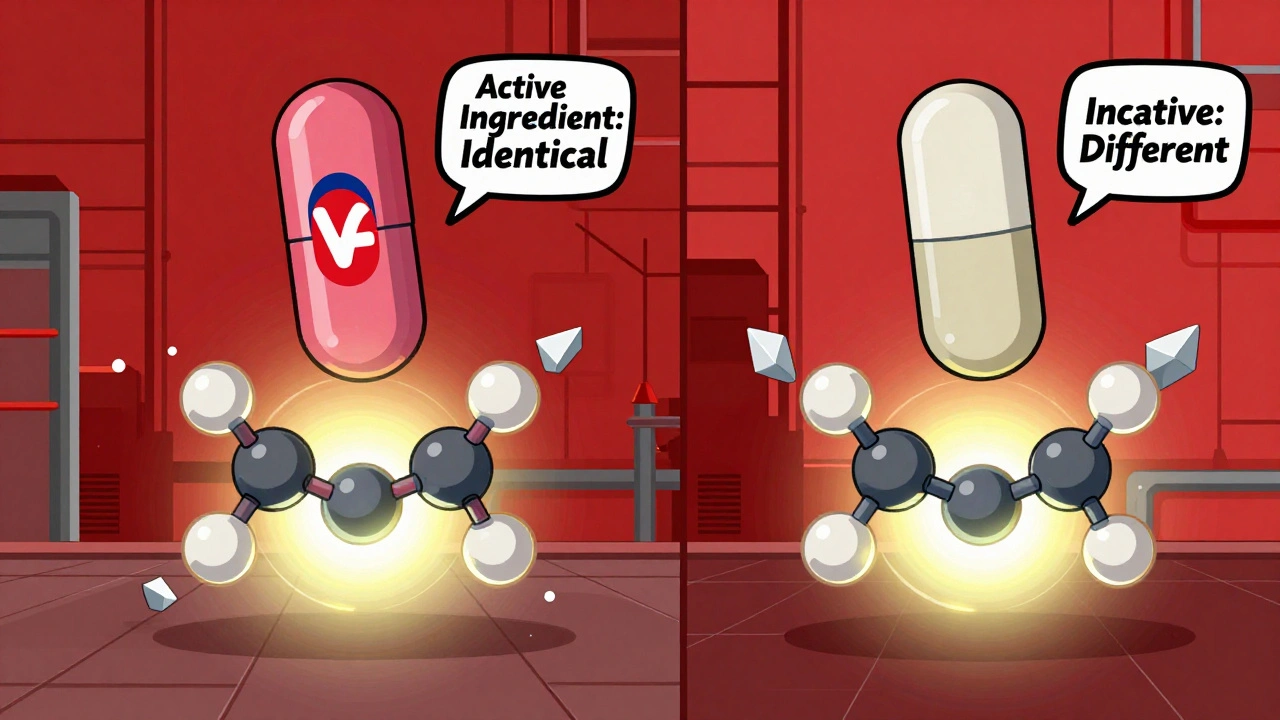
Are Generic Drugs Copies? The Truth Behind the Myth
Generic drugs are not copies - they're FDA-approved equivalents with the same active ingredients as brand-name medications. Learn the science behind why they work the same, cost 85% less, and are safe for most people.
Continue reading