SEARCH
Generic vs Brand: What Really Matters When Choosing Medications
When you pick up a prescription, you might see two options: the familiar brand name or a cheaper generic. But are they truly the same? The answer isn’t just yes or no—it’s about generic vs brand, the difference in cost, perception, and regulatory standards between name-brand and non-brand medications. Also known as brand-name drugs, these are the original formulations developed by pharmaceutical companies, while generic drugs are copies approved by the FDA to work the same way, with the same active ingredients, strength, and dosage form. What most people don’t realize is that over 90% of prescriptions filled in the U.S. are for generics—and they save patients and the system billions every year.
The real question isn’t whether generics work—it’s why so many still doubt them. therapeutic equivalence, a system used by the FDA to rate how closely a generic matches its brand-name counterpart. Also known as TE codes, these ratings tell pharmacists which substitutions are safe and effective. A drug with an AB1 rating means it’s bioequivalent to the brand and can be swapped without changing how your body responds. But here’s the twist: authorized generics, the exact same pill as the brand, just without the marketing label. Also known as brand-name drugs, they’re made by the same company, in the same factory, on the same line—just sold under a different name to undercut the original price. You’re getting the identical product, often for half the cost.
Some people notice differences in how a generic feels—maybe it causes slightly more nausea or doesn’t seem to kick in as fast. But that’s rarely because the medicine itself is different. More often, it’s because the inactive ingredients (fillers, dyes, coatings) vary between manufacturers. These don’t affect how the drug works, but they can change how it dissolves or how your stomach reacts. That’s why switching between different generic brands might feel different, even though both are FDA-approved. The key is consistency: if you find a generic that works for you, stick with it. Don’t let pharmacy substitutions confuse you.
What’s surprising is how much of the price gap comes from branding, not biology. Brand-name companies spend millions on ads, fancy packaging, and sales reps. Generics skip all that. Authorized generics skip even more—they don’t need to invest in building trust because they’re the real thing, just unlabeled. And when you see a drug priced wildly differently overseas, like in Switzerland versus India, it’s not about quality—it’s about policy, patents, and who’s paying.
There’s no magic here. No hidden science. No secret formula. Just clear rules, solid data, and a system designed to give you the same medicine at a fair price. The FDA doesn’t approve generics because they’re "good enough." They approve them because they’re proven to be identical in performance. The real barrier isn’t science—it’s perception. And that’s something you can change, starting today.
Below, you’ll find real stories, clear explanations, and practical advice on how to save money without risking your health—from decoding the Orange Book to spotting fake pills that look just like the real thing. You’ll learn how to ask your pharmacist the right questions, when to insist on an authorized generic, and why your insurance might push you toward a specific version. This isn’t theory. It’s what people are actually using to cut their drug bills in half.
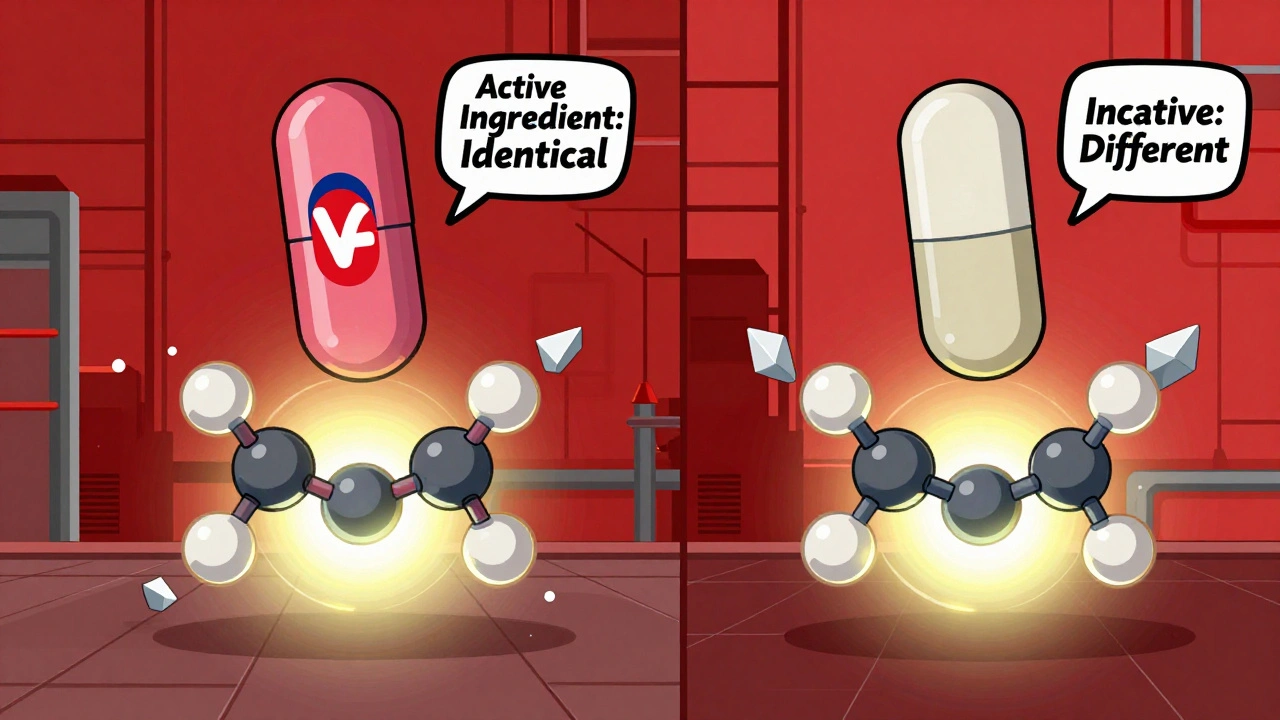
Are Generic Drugs Copies? The Truth Behind the Myth
Generic drugs are not copies - they're FDA-approved equivalents with the same active ingredients as brand-name medications. Learn the science behind why they work the same, cost 85% less, and are safe for most people.
Continue reading