SEARCH
FDA: What It Does, Why It Matters, and How It Keeps Your Medicines Safe
When you pick up a bottle of FDA, the U.S. agency responsible for approving and monitoring drugs, medical devices, and food safety. Also known as the Food and Drug Administration, it’s the reason your pills have clear labels, your generics work just like brand names, and fake drugs are (mostly) kept off shelves. You don’t see it, but the FDA touches every medication you take—from the antibiotic you get for an infection to the painkiller you buy without a prescription.
The generic drugs, medications approved by the FDA as identical in active ingredients, strength, and effect to brand-name versions you save money on? They had to pass the same rigorous tests as the original. The OTC Drug Facts, the standardized label on every over-the-counter medicine that tells you exactly what’s inside, how to use it, and what to avoid? That’s the FDA’s doing too. And when counterfeit pills with deadly fentanyl slip into the market, it’s the FDA’s warnings and partnerships with law enforcement that try to stop them before they reach your medicine cabinet.
It’s not perfect. Drug prices still vary wildly across countries, and not every patient trusts generics—even though they’re the same medicine made by the same factory. But the FDA’s rules are what make batch release testing, statin monitoring, and antibiotic warnings possible. Without it, there’d be no way to know if your warfarin interacts with that new antibiotic, or if your new Zoloft is actually Zoloft. The FDA doesn’t just approve drugs—it enforces trust. And that trust is what lets you take a pill without wondering if it’ll kill you instead of help you.
Below, you’ll find real stories and facts about how the FDA shapes what’s in your medicine bottle—whether it’s stopping fake pills, explaining why authorized generics cost less, or how a simple OTC label can prevent an overdose. These aren’t theory pieces. They’re what happens when regulation meets real life.
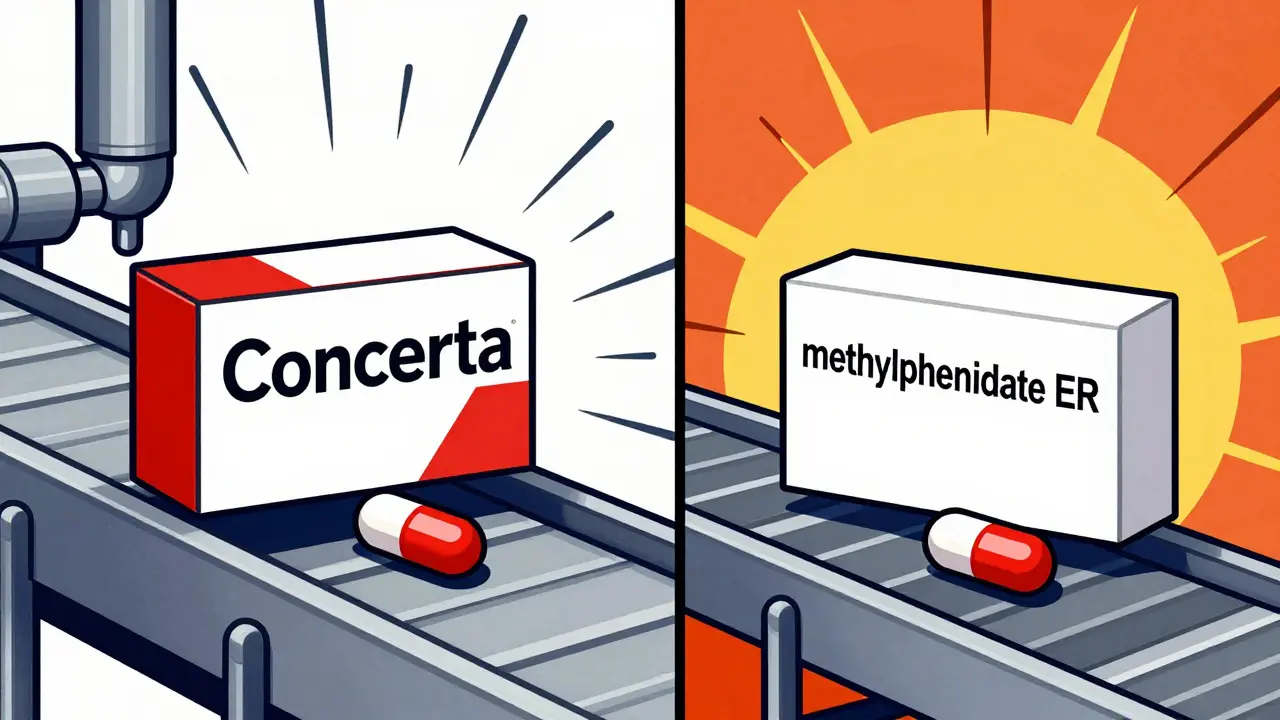
What Are Authorized Generics? Complete Explanation
Authorized generics are identical to brand-name drugs in every way-same ingredients, same manufacturer, same factory-just without the brand label. Learn how they work, why they exist, and how they can save you money without compromising quality.
Continue reading
The Orange Book: How Therapeutic Equivalence Guides Generic Drug Substitution
The Orange Book is the FDA's official guide to therapeutic equivalence, helping pharmacists determine which generic drugs can safely replace brand-name medications. Learn how TE codes work, why they matter, and how they save billions in healthcare costs.
Continue reading